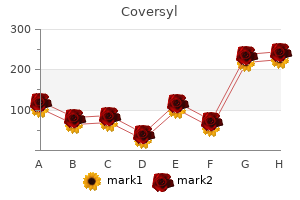
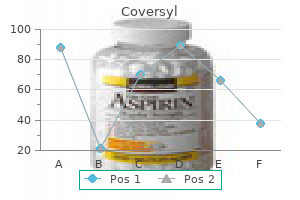
Park W. Willis IV, MD
Therefore it is necessary to frst construct a killing curve which will inform the researcher of the conditions required for bacterial killing symptoms night sweats order on line coversyl. However medicine for stomach pain cheap 8 mg coversyl visa, the isolation of the individual is not necessary since humantohuman transmission of the disease has not been reported fungal nail treatment buy coversyl without prescription. For details of chemotherapeutic regimes for the treatment of tularaemia see chapter 5 treatment regimen discount 4 mg coversyl visa. This vaccine would also have a utility for the protection of researchers who are working with highlyvirulent strains of F. It is also unavailable in Europe and most other parts of the world, but a live attenuated vaccine is still in use some parts of the former Soviet Union 43 wHo guidelines on tulArAemiA where it has been used to immunize millions of people against tularaemia (Sjostedt, Tarnvik & Sandstrom, 1996). Of the animal species which have been evaluated as models of disease, the nonhuman primates and rabbits appear to mimic human disease most accurately. In contrast, mice appear to be uniformly susceptible to disease caused by strains belonging to all subspecies, including F. This model of disease is valuable because protective immune responses can be induced by the delivery of this strain intradermally or by scarifcation while mechanisms of virulence can be investigated when the strain is given by the intraperitoneal or intravenous routes. There is also good reason to believe that these fndings refect the nature of the protective responses in humans; previous studies have shown that the passive transfer of immune serum provides only very limited protection against tularaemia. In addition, different batches of the vaccine show different immunogenicity, a property ascribed to the different proportions of socalled blue and grey colony types (Eigelsbach & Downs, 1961). There is some evidence that the vaccine is effective when given by the airborne and oral routes of delivery (Saslaw et al. All of the control subjects developed tularaemia and at the earliest indication of disease were treated with streptomycin or tetracycline. The majority of vaccinees challenged with up to 2000 organisms escaped major clinical illness. The incidence of ulceroglandular tularaemia was unchanged but the clinical signs and symptoms of this form of the disease were moderated in vaccinated individuals. Vaccination of laboratory workers may reduce the risk of laboratoryacquired infections (Rusnak et al. However, the data presented above indicate the value of this vaccine for the prevention of laboratory acquired tularaemia. Similarly, many strains are resistant to erythromycin, although this marker may be useful in erythromycinsusceptible strains. Because the tetracyclines are often used as drugs of frst choice, the use of tetracycline resistance as a marker may not be acceptable in all countries. Additionally, care should be taken over the selection of kanamycin resistance markers. It is important to use a gene that encodes kanamycinspecifc resistance rather than one which provides resistance to a range of aminoglycosides since this might provide resistance to streptomycin and gentamicin. The kanamycin resistance gene found in transposon Tn5 is kanamycinspecifc and can be used in F. In some countries additional legislation operates to regulate the acquisition of strains of F. The stringent controls on access are currently the subject of considerable discussion. Infuence of genetic background on host resistance to experimental murine tularemia. Regionalization of tularemia foci in the tugai type on a zoo geographical and epizootiological basis. Antimicrobial susceptibility testing of Francisella tularensis with a modifed MuellerHinton broth. The pathology of untreated and antibiotic treated experimental tularemia in monkeys. Chronic shedding tularemia nephritis in rodents: possible relation to occurrence of Francisella tularensis in lotic waters. Early recognition of atypical Francisella tularensis strains lacking a cysteine requirement. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Evaluation of a safraninOstained antigen microagglutination test for Francisella tularensis antibodies. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory acquired tularemia. Missed sentinel case of naturally occurring pneumonic tularemia outbreak: lessons for detection of bioterrorism. Reconstruction and prognosis of water vole population dynamics on the basis of tularemia morbidity among Novosibirsk oblast residents. The 2000 tularemia outbreak: a casecontrol study of risk factors in diseaseendemic and emergent areas, Sweden. Streptomycin and alternative agents for the treatment of tularemia: review of the literature. Tularemia: a summary of certain aspects of the disease including methods for early diagnosis and the results of serum treatment in 600 patients. A comparative study of the treatment of tularemia with immune serum, hyperimmune serum, and streptomycin. Problems in identifcation of Francisella philomiragia associated with fatal bacteremia in a patient with chronic granulomatous disease. A rapid, highly sensitive method for the detection of Francisella tularensis in clinical samples using the polymerase chain reaction. Serological survey for diseases in freeranging coyotes (Canis latrans) in Yellowstone National Park, Wyoming. A procedure for differentiation between the intentional release of biological warfare agents and natural outbreaks of disease: its use in analysing the tularemia outbreak in Kosovo in 1999 and 2000. Tickborne oculoglandular tularemia: case report and review of seasonal and vectorial associations in 106 cases. Detection of Francisella tularensis in infected mammals and vectors using a probebased polymerase chain reaction. Water and airborne Francisella tularensis biovar palaearctica isolated from human blood. Tularemia in a captive goldenheaded lion tamarin (Leontopithecus chrysomelas) in Switzerland. Cycles in voles and small game in relation to variations in plant production indices in northern Sweden. Frequent isolation of Francisella tularensis from Dermacentor reticulatus ticks from an enzootic focus of tularaemia. Prevalence of Francisella tularensis in Dermacentor reticulatus ticks collected in adjacent areas of the Czech and Austrian Republics. Tularemia; geographical distribution of deerfy fever and the biting fy, Chrysops discalis Williston.
Virological and serological studies of Venezuelan equine encephalomyelitis in humans medicine glossary purchase 8 mg coversyl otc. The systemic pathology of Venezuelan equine encephalitis virus infection in humans treatment zygomycetes purchase coversyl 4 mg amex. Venezuelan equine encephalitis febrile cases among humans in the Peruvian Amazon River region symptoms glaucoma buy coversyl 4mg visa. Aerosol infection of cynomolgus macaques with enzootic strains of venezuelan equine encephalitis viruses medicine dictionary pill identification generic coversyl 8 mg without prescription. Hemorrhagic fever viruses as biological weapons: medical and public health management. Air evacuation under highlevel biosafety containment: the aeromedical isolation team. Safe intensivecare management of a severe case of Lassa fever with simple barrier nursing techniques. Lassa fever in the United States: investigation of a case and new guidelines for management. Update: Filovirus infections among persons with occupational exposure to nonhuman primates. Update: Management of patients with suspected viral hemorrhagic fever United States. Botulism surveillance and emergency response: a public health strategy for a global challenge. Investigation of a ricincontaining envelope at a postal facility South Carolina, 2003. Production and purification of a recombinant Staphylococcal enterotoxin B vaccine candidate expressed in Escherichia coli. Rapid and sensitive sandwich enzymelinked immunosorbent assay for detection of staphylococcal enterotoxin B in cheese. Multitoxin biosensormass spectrometry analysis: a new approach for rapid, realtime, sensitive analysis of staphylococcal toxins in food. Hamaki T, Kami M, Kishi A, Kusumi E, Kishi Y, Iwata H, Miyakoshi S, Ueyama J, Morinaga S, Taniguchi S, Ohara K, Muto Y. Vesicles as initial skin manifestation of disseminated fusariosis after nonmyeloablative stem cell transplantation. Mycotoxins (trichothecenes, zearalenone and fumonisins) in cereals associated with human redmold intoxications stored since 1989 and 1991 in China. Comparative study on the natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in corn and wheat from high and lowrisk areas for human esophageal cancer in China. A survey of Fusarium toxins in cerealbased foods marketed in an area of southwest Germany. Addressing Emerging Infectious Disease Threats: A Prevention Strategy for the United States. The structure and receptorbinding properties of the 1918 influenza hemagglutinin, Science 2004 303:18381842. Comparison of a commercial enzymelinked immunosorbent assay with immunofluorescence and complement fixation tests for detection of Coxiella burnetii (Q fever) immunoglobulin M. Application of rapidcycle realtime polymerase chain reaction for the detection of microbial pathogens: the MayoRoche Rapid Anthrax test. National Response Team Technical Assistance for Anthrax Response, InterimFinal Draft, Sep 2002, pp2223, wetp. National Response Team Technical Assistance for Anthrax Response, InterimFinal Draft, Sep 2002, pp5359, wetp. A physician could decide to prescribe cidofovir for an individual case of generalized Vaccinia. In that situation, the physician assumes the legal risk as would occur with any medical intervention. Clinical trials are research studies designed to determine the safety and effectiveness of the drug in people. This application is carefully reviewed and, if the drug is found to be reasonably safe and effective, it is approved. Because these diseases occur in humans rarely, it is impossible to test the effectiveness and it is unethical to challenge humans with potentially deadly disease just to test a new drug. All recipients of the product (subjects) must meet specific eligibility criteria and acknowledge having received informed consent with their signature before receiving the product. Therefore, participation in the protocol is voluntary and cannot be required or coerced. This informed consent requirement can only be released by a presidential waiver, under very special and limited circumstances. A mandatory requirement for the investigational use of a product is documentation of the administration of the product, with strict accountability of product shipment, storage conditions, and for any doses that were given. Instructions for Receipt and Administration of Investigational Drugs for Military Healthcare Providers 1. Alternatively, the patient could be evacuated to the nearest medical center with a pretrained, designated investigator who will administer the product. Instructions for Receipt and Administration of Investigational Drugs for Civilian Healthcare Providers 1. Civilian healthcare providers should first contact their state health departments for guidance. L3 Appendix M: Use of Drugs / Vaccines in Special or Vulnerable Populations in the Context of Bioterrorism. Some vaccines, even though licensed for use in children, are more problematic in children than in adults. Smallpox vaccine is much more likely to lead to postvaccinial encephalitis, an oftenfatal condition, when given to young children. Yellow fever vaccine is more likely to cause severe encephalitis in young infants than it is in adults. Some antimicrobials are relatively contraindicated in children due to real or perceived risks which do not appear to be present in adult populations. This class of antibiotics is generally contraindicated in children less than 8 years old because the antibiotic and its pigmented breakdown products can cause permanent dental staining and, more rarely, enamel hypoplasia during odontogenesis. The degree of staining is proportional to the total dose received and is thus dependent upon both dose and duration of therapy. Thus, doxycycline, which is given only twice per day, represents a lower risk than other tetracyclines. Tetracyclines may also cause reversible delay in bone growth rate during the course of therapy. Rocky Mountain spotted fever and other rickettsial diseases), specifically including treatment or prevention of anthrax disease. This class of antibiotics is generally contraindicated in patients less than 18 years old because it is associated with cartilage damage in juvenile animal models. While sporadic cases of arthropathy in humans have been reported, they have primarily been associated with adults and children receiving pefloxacin, a fluoroquinolone commonly used in France. Ciprofloxacin, which has been used extensively in children, has not thus far been associated with arthropathy and seems to be well tolerated. If the organism is later determined to be susceptible to penicillins, then one could switch to amoxicillin to complete the course of antibiotics. If the organism is not susceptible to penicillin but is susceptible to doxycycline and ciprofloxacin, then ciprofloxacin may represent a better choice for continued prophylaxis, as arthropathy from fluoroquinolones thus far has proved rare in children, whereas the necessarily prolonged course of doxycycline (perhaps 60 days) could lead to significant dental staining. If the same child was exposed to Yersinia pestis susceptible to both ciprofloxacin and doxycycline, doxycycline might be an equally good choice as ciprofloxacin, as the short (7 day) course of postexposure prophylaxis is unlikely to result in dental staining. Antimicrobial doses are often different in children, and prescribed according to patient weight. Some representative antibiotics and their pediatric doses are included in Table 1. Nursing mothers Some medications are excreted in breast milk (see Table 1), and thus may be ingested by nursing infants. Such medications, if contraindicated in infants and orally absorbed, should also be avoided by breastfeeding mothers if possible.
Taking tacrolimus with other immunosuppressive medicines for a long time may increase your risk for getting a serious infection and also increases your risk for lymphoma symptoms parkinsons disease cheap 4 mg coversyl free shipping. You and your doctor will consider the risks and the benefits to choose the best plan for you treatment meaning generic coversyl 4 mg without prescription. Original: September 30 medications versed purchase cheapest coversyl, 2009 Page 74 Revised: June 19 medicine head buy coversyl with a mastercard, 2019 Inflammatory Bowel Disease Program Patient Information Guide Are there medicines I should avoid while taking tacrolimusfi Nonprescription products: Do not eat grapefruit or drink grapefruit juice or alcohol while taking tacrolimus. Ask your doctor or pharmacist if your other medicines are safe to take with tacrolimus. Common medicines to avoid while taking tacrolimus include antacids, Prozac (and other serotonin reuptake inhibitor antidepressants), fluconazole (and other antifungals), and triamterene (and other potassium sparing diuretics). Other medicines to avoid while taking tacrolimus include cyclosporine, dabigatran etexilate, natalizumab, protease inhibitors, rifamycin derivatives, sirolimus, or temsirolimus. Tacrolimus is metabolized (broken down) by the cytochrome P450 enzyme system, so any medicine that interferes with this system will change the effects of tacrolimus in your body. There are several of these kinds of medicines so be sure to tell your doctor about every prescription and overthecounter medicine you are taking. This includes vitamins and herbal products as well as medicines prescribed by other doctors. You will have routine blood tests to check your electrolytes, such as potassium and magnesium, and your liver and kidney function, which may be affected by tacrolimus. The level of tacrolimus in your blood will be measured, as well as the levels of inflammatory markers and lipids. It is common to have your blood test done 3 times a week at first and then less often as time goes by. Your blood pressure and blood glucose level will also be checked at the same time. Be sure to tell your doctor about any other medicines you are taking because they may affect the level of tacrolimus in your body. Allergic reaction: You are unlikely to have an allergic reaction to tacrolimus when you first start taking it. Anaphylactic shock, where you faint or lose consciousness (vascular shutdown), is rare. Common side effects include headache, dizziness, tremor, numbness, tingling, trouble sleeping (insomnia), weakness, abdominal pain, constipation, diarrhea, stomach upset, nausea, vomiting, anemia, elevated or low white blood cell count, low platelets, and joint pain. Kidney: Poor kidney function, including kidney damage, may occur when tacrolimus is used at high doses. Liver: Levels of liver enzymes and bilirubin may rise when tacrolimus is used at high doses. Hypertension: Your blood pressure will be monitored closely while you are taking cyclosporine. Seizures: If you have low lipid (blood fat) levels you may be at risk for seizures. Lymphoma: Because tacrolimus is an immunosuppressive medicine there is a small risk for getting lymphoma, which is a type of cancer. Tell your doctor right away if you notice any increase in pain, weight loss, or ongoing fevers you cannot explain. Infections: There is an increased risk for infection, such as Pneumocystis carinii pneumonia. This risk is higher if you are taking another immunosuppressive medicine while you are taking tacrolimus. This information is not meant to cover all uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. Original: September 30, 2009 Page 76 Revised: June 19, 2019 Inflammatory Bowel Disease Program Patient Information Guide Mycophenolate What is mycophenolate and how does it workfi It is used to treat ulcerative colitis that does not respond to standard medicines. It is most often used by people who have had a kidney, liver, or heart transplant. Mycophenolate blocks the increase in the numbers of white blood cells called T cells and B cells, in order to reduce inflammation. As mentioned, mycophenolate is an immunosuppressive medicine, which means it partially blocks the action of the immune system, but it does not completely turn it off. While there are some side effects, most people do not get more infections when they start taking this medicine. Some people who cannot tolerate or do not improve with the immunosuppressive medicines commonly used to treat ulcerative colitis, such as azathioprine (Imuran), methotrexate, or infliximab (Remicade). If you improve while taking mycophenolate, you will have the benefit of limiting the time you need to take to corticosteroids like prednisone. In addition, you may be able to avoid the complications of untreated inflammation that can lead to surgery. Do not let the powder touch your skin or the moist lining of your mouth, nose, and eyes. Do not take it 1 hour before or 2 hours after taking an antacid or a cholestyramine medicine (for example, Questran). If you take the delayed release tablets, these should not be crushed, cut, or chewed. If you are taking oral mycophenolate, do not change the dose or stop taking this medicine without talking to your doctor. Drink plenty of fluid (2 to 3 quarts every day as long as you are taking this medicine, unless you have been told to limit fluids. Taking mycophenolate with other immunosuppressive medicines for a long time may increase the risk for serious infections. You and your doctor will consider the risks and the benefits to choose the best plan for you Are there medicines I should avoid while taking mycophenolatefi Prescription medicines: Common medicines to avoid while taking mycophenolate include antacids and birth control pills (oral contraceptives). Also, do not take azathioprine, cholestyramine resin, cyclosporine, magnesium salts, metronidazole, natalizumab, norfloxacin, probenecid, rifamycin derivatives, or sevelamer. If you have diabetes you need to check your blood glucose levels often with your home glucose monitor. We often check blood counts regularly as mycophenolate can reduce the white blood cell count. Allergic reaction: You are unlikely to have an allergic reaction to mycophenolate when you first start taking it. This risk is higher if you are taking another immunosuppressive medicine while you are taking mycophenolate. You should have a working thermometer at home to measure your temperature when you are sick. Patients with fever, cough, malaise (general sick feeling), difficulty breathing, or new or increasing fatigue need to see their doctor right away. Neutropenia: Neutropenia is a decrease in the numbers of the white blood cells called neutrophils. Lymphoma: Because mycophenolate is an immunosuppressive medicine, there is a small risk for getting lymphoma, which is a type of cancer.
This is the disease is prolonged medicine 7 order 4mg coversyl amex, with even complete remitters having particularly relevant if the nephrotic syndrome is severe 10 medications doctors wont take cheap coversyl 4 mg on line, since a relapse rate of up to 40% treatment plantar fasciitis buy genuine coversyl on line. A retrospective observational study compared highdose There are no data to support treatment with corticosteroids oral prednisone (1 mg/kg/d) for at least 4 months and in patients without nephroticrange proteinuria and treatment using drugs buy coversyl with a mastercard, tapering thereafter, with lowdose prednisone (0. Lowdose prednisone was given to 16 patients 165 resistant disease with poor outcome. Remission rates tional studies conducted after 1985 have reported better were comparable; 63% for prednisone (n fi 9), 80% for outcomes and suggested that this improvement in response prednisone plus azathioprine (n fi 6), and 86% for predni was associated with a higher initial dose and longer duration 172 sone plus cyclosporine (n fi 10). Spontaneous remissions do occur, with reported observed in the two regimens, 71% (12/17 patients) vs. These limited data suggest that more likely to occur in patients with tip lesions, with pre patients who do not tolerate prolonged highdose pred 179 served kidney function, and lower grades of proteinuria. If no remission by 6 months, discontinue cyclosporine of prednisone therapy that defines steroidresistance. K Relapses are very frequent after withdrawal of cyclo the variation in reported remission rates may depend on the sporine. More prolonged treatment may lead to more definition of steroid resistance, the prior use of alkylating persistent remissions. Relapses occur frequently when agents, and the concomitant use of lowdose prednisone. Case reports and small observational studies have the consequences of any such inaccurate or misleading data, reported response to alkylating agents, sirolimus, and ritux opinion or statement. Detailed morphological studies show mesangial features include capillary wall thickening, normal cellularity, deposits by electron microscopy and prominent IgG1, 2, or IgG and C3 along capillary walls on immunofiuorescence, 3 subclass deposits by immunofiuorescence in secondary and subepithelial deposits on electron microscopy. Etiology and clinical characteristics of membranous nephropathy in Chinese patients. K There is lowquality evidence to support a recommenda the degree and persistence of proteinuria during a period of tion that the period of observation may be extended in observation helps in selecting patients for this therapy. Dermatomyositis Schistosomiasis Ankylosing spondylitis Filariasis Partial Remission: Urinary protein excretion o3. Treatmentinduced Probenicid a1antitrypsin deficiency 221, 222 remissions are associated with an improved prognosis. Trimethadione WeberChristian disease Nonsteroidal antiinflammatory Primary biliary cirrhosis the 10year survival free of kidney failure is about 100% in drugs Systemic mastocytosis complete remission, 90% in partial remission, and 50% with Cyclooxygenase2 inhibitors GuillainBarre syndrome no remission. Patients with complete or partial remission Clopidogrel Urticarial vasculitis have a similar rate of decline in CrCl: A1. Those with a persistent observational studies and has been observed only in those nephrotic syndrome are also exposed to the related patients with proteinuria (o10 g/d) at baseline. Both is dependent upon the age, gender, degree of proteinuria, and agents were of comparable efficacy, reducing proteinuria on 216, 217 kidney function at presentation. In comparative studies, 6month cyclical regimen of alternating alkylating agents cyclophosphamide has a superior safety profile compared (cyclophosphamide or chlorambucil) plus i. Quality of life, as measured by a especially in the absence of massive proteinuria, any visual analog scale, was significantly better in the treatment acceleration of the rate of decline indicates the possibility group throughout the followup period. The complication of a superimposed disease process (such as crescentic rate was not different in the two groups. This study agrees with treated (n fi 42) and 60% of the control (n fi 39) patients earlier observational studies from the same authors, and were alive with normal kidney function (P fi 0. However, toxicity with this specific approach has (5% complete remission) in the two groups. A significantly higher proportion of patients in the adverse effects of alkylatingcytotoxic agents are substantial, chlorambucil arm were in remission in the first 3 years. The and include gonadal toxicity, bladder carcinoma, bone difference was lost at 4 years, probably because of a small marrow hypoplasia, leukemogenesis, and serious opportu number of atrisk cases. The available evidence does not K Studies are needed to determine the value of renal suggest a beneficial effect of i. A rapid men or who have contraindications to this deterioration of kidney function in the absence of massive regimen. The current evidence is insufficient to make any specific recommendation in this group of dosage recommendations. The results plus cyclosporine and compared to placebo plus predni indicated no difference between treatments in terms of 249 sone. Complete and partial remissions in proteinuria were partial or complete remission of proteinuria (79% vs. Relapses cyclosporine was discontinued was high, approximately 45% occurred in approximately 15% of both groups. Regular midebased regimens may be preferred in this situation, but monitoring of cyclosporine blood concentration as well as dose reduction of the alkylating agent is advisable. The probability of a complete or partial alone, in either induction of remission or preservation remission did not differ between the two groups after of kidney function, even after the data were adjusted to 12 months. Nevertheless, retrospective studies conducted in comparable efficacy to the standard regimen of cyclical subjects of Asian (Japanese) ancestry have suggested possible alkylating agents and steroids, the present evidence is 262 benefits for steroid monotherapy. At 1 year, the proportion of patients who achieved disease remission was identical to that of 24 initial therapy. Among 18 patients who completed 24 months of therapy followup, four achieved complete remission, 12 achieved 7. It showed a significant reduction 251 in the rate of loss of kidney function with cyclosporine. However, the same therapy that resulted in the initial adverse effects of treatment may be more frequent in patients remission. Rapidly to retreatment of a relapse are similar to those observed after progressive renal failure may occur from an acute hypersen the first treatment. Cancer induction is a major concern when alkylating matic, as they seem to have a higher spontaneous remission agents are used for an extended period. Purchase coversyl 8 mg visa. The H1N1 Swine Flu: A Look Inside. |