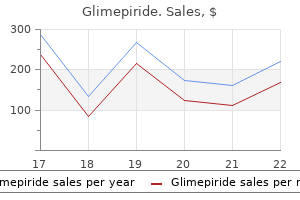
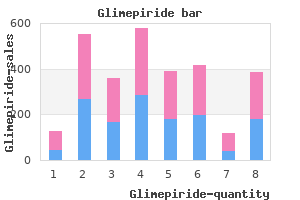
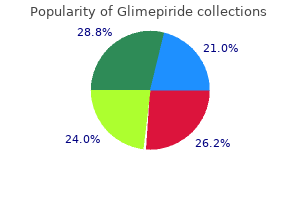
Markus Frey, MD
With the diverse representation of women via the alternative and mainstream media diabetes mellitus complications cheap 3mg glimepiride, it not only minimises stereotypes but also portrays all females to be uniquely beautiful diabetes mellitus definition by ada purchase 3 mg glimepiride otc. Therefore blood glucose units conversion purchase glimepiride uk, the data analysis argues that media is not always an oppressive tool which harms self-esteem or perpetuates body dissatisfaction among individuals metabolic disease symposium buy glimepiride 3mg visa. However, my data analysis in response 28 29 to Questions 7 and 8 disagrees with the literature reviewfis statement. According to my data analysis, 56 participants (51 percent) and 23 participants (21 percent) answered Question 8 that they are mostly satisfied or extremely satisfied with their physical features. Whereas in response to Question 7, 72 out of 110 participants (65 percent) answered they believe they are beautiful. Therefore, my data analysis proves the participants of the structured interview are likely to be not only satisfied with their bodies but also might have high self-esteem. However, I believe in-depth studies would be useful to investigate the relationship between body dissatisfaction and self-esteem among individuals in the near future. The literature review mentions about the overemphasis of Western ideal of beauty which denies the value of non-Western beauty (Halprin, 1995). My data analysis confirms with the literature reviewfis statement by providing an example of my interview participant explaining the use of unrealistic portrayals of non-Western models in non-Western media and advertising. For example, the use of ideal models with fair complexion and big eyes in Asian advertising which Grogan (2008) believes leave Asian consumers experiencing low self-esteem and appearance anxiety. The data analysis also agrees with the literature review that the ingrained white beauty standard is detrimental to non-Western consumersfi selfesteem. Although both the data analysis and literature review have touched on the effects of 28 Question 7Do you think you are beautifulfi In addition, an in-depth study on portrayals of non-Western women in both Western and non-Western media and advertising would be useful in the fields of advertising, media and communication studies. According to the literature review, a womanfis body can be a tool of resistance against the objectification of the body in consumer culture (Gimlin, 2002). The data analysis agrees with the literature reviewfis statement by confirming that women have better self-esteem towards themselves when various female bodies are accepted by media and society. Although the literature review says gaining an insight into the beauty culture helps us to think critically towards feminine beauty, it does not explain further how we, as individuals, can play a role in improving womenfis self esteem in society. The data analysis mentions that compliments, support and encouragement from peers and family are vital in improving womenfis self-esteem as well as minimising the chances of experiencing body dissatisfaction. Therefore, the data analysis proves that pro-woman support and views towards beauty equate higher self-esteem among females. Although the data analysis has explored further into self-esteem by confirming its correlation with body satisfaction as mentioned in the literature review, I believe further research into self-esteem would be useful to enable us to understand body dissatisfaction among women in-depth in the fields of psychology and communication studies. From the Rubenesque body to the present-day supermodel, the literature review confirms that ideal beauty varies across time and cultures (Mazur, 1986). My data analysis agrees with Lin and Yehfis (2009) statement by confirming 95 percent of the 110 30 participants have responded to Question 11A of the structured interview by naming wellknown women of their choice whom they believe epitomize feminine beauty. The data 31 analysis also investigates further where participants respond to Question 11B by revealing qualities their feminine icons have which they believe relate to feminine beauty. My literature review confirms that consumers would strive to discipline their bodies in order to match the ideals displayed on print and screen. Both the literature review and data analysis highlight the overrepresentation of the idealized Western beauty through media and advertising especially towards non-Western consumers (Halprin, 1995). For example, the frequent use of models with pale skin or Eurasian features to endorse beauty products in Asian advertising which Makkar and Strube (1995) believes it not only denies the existence of real Asian women but also fuels the unrealistic ethnic-based standards of beauty ideals. The overrepresentation of Western beauty not only makes the beauty ideal socio-culturally homogenous but also leave non-Western consumers experiencing low self-esteem and feeling dissatisfied towards their bodies (Grogan, 2008). Darling-Wolf (2004) and Blum (2007) argue that consumers can negotiate with media influences whilst expressing their uniqueness amidst a globalised consumer culture without 80 having to refashion themselves as celebrity-grade humans. Therefore, the data analysis confirms that proper depictions of femininity not only can lower womenfis anxiety in fulfilling the beauty ideal but also minimize the stereotypical portrayal of women in advertising and media. Body image According to Oberg and Tornstam (1999), body image is fundamental to individuals where self-identity is affected by judgments made towards the body. Although the literature review states body image is believed to be related to physical and mental health, Thompson (1999, cited by Grogan, 2008) defines body image as an individualfis perceptions and feelings towards his or her body. Body image is also determined by social experiences such as exposure to media or socio-cultural cues and peer influence. With the internalization of the unattainable ideal, Groesz, Levine and Murnen 81 (2002) believe body dissatisfaction is likely to lead to low self-esteem, eating disorders and depression. Grogan (2008) adds that body dissatisfaction, especially within the sports culture, can lead to drug abuse and over-exercising when an athlete attempts to fulfil the athletic ideal. However, the data analysis argues that individuals who reject the beauty ideal are likely to have positive views towards their bodies including healthy eating habits and higher selfesteem. My data analysis confirms its statement when 79 participants (72 percent) of the structured interview are reported to be satisfied with their bodies in response to Question 32 8. The data analysis also discovers that 72 interview participants (65 percent) believe they 33 are beautiful in response to Question 7. With body image issues on the rise, future research into body dissatisfaction should not only just explore weight and appearance concerns but also investigate how some individuals maintain positive body image by resisting the beauty ideal (Dione, Davis, Fox, and Gurevich, 1995). Grogan (2008) includes the importance of further research into body image and self-esteem issues among nonWestern groups. Beauty practices (Historical and Contemporary) the thesis chapter (Historical and Contemporary Beauty Trends) investigating Western and non-Western beauty practices, both historical and contemporary, helps us to understand how women endure pain, labour and pressure to regulate their bodies in order to achieve the ideal standard of beauty. In the literature review, Jeffreys (2005) believes that beauty practices are harmful and aim to create a stereotyped femininity which portray women spending amounts of time and energy in order to fulfil the accepted standard of attractiveness. However, Bloch and Richins (1992) argue that beauty practices are not necessarily oppressive where they can serve as positive selfesteem enhancers. For instance, the use of cosmetic concealers which are found to boost the self-esteem of individuals with facial blemishes (Wright et. As little is known about consumer behaviour with regard to beauty practices, additional research is needed to not only understand the roles of beauty practices in societies but also the investigation of relations between consumption and self-esteem. The literature review agrees when the body is under constant surveillance and scrutiny, the individual would be considered to have failed to attain perfection through diet and exercise as means of discipline and control. When diet and exercise fail, the literature review proves how some individuals would make do with cosmetic surgery or other beauty practices as a last resort to achieve the perfect body (Gimlin, 2002).
Assistance is available for eligible patients regardless of their insurance type with commercial insurance (such as the one Nurses do not provide medical advice and are trained to direct people with cancer and their caregivers to speak you get through your employer) diabetes medications algorithm generic glimepiride 3 mg fast delivery, public insurance (such as Medicare or Medicaid) metabolic disease icd 9 discount 2 mg glimepiride mastercard, or no insurance diabetes symptoms and feet discount glimepiride 2mg overnight delivery. Patients using Medicare diabetes type 2 news buy generic glimepiride, Medicaid, or any other federal or state government program to Talk to your healthcare provider if you have concerns about fertility. After reaching the maximum Program beneft, the patient will be responsible for all out-of-pocket costs. All participants are responsible for reporting the receipt of all Program benefts as required by any insurer or by law. No party may seek reimbursement for all or any part of the beneft received through this Program. Genentech reserves the right to rescind, revoke, or amend the Program without notice at any time. If you when you frst start treatment and during treatment with this may affect your ability to father a child. It is important to keep your appointments for healthcare provider if you have concerns about fertility. You are encouraged to report negative side effects of tell your healthcare provider right away. Follow the checklist below to get started with your treatment Confrm how you will start treatment Talk with your healthcare provider to fnd out when and where you will start your treatment. Confrm delivery method Talk with your healthcare provider to determine how to obtain your medication. Get additional information about living with leukemia* the Leukemia & Lymphoma Research American Cancer Society Lymphoma Society Foundation The organizations listed above have not endorsed AbbVie and Genentech or any of their respective products or services. AbbVie and Genentech have listed these organizations only as a convenience and according to the permissions granted by their respective terms of use. Each organization has its own terms and conditions and privacy policy that you should read if you choose to contact any of them. Other studies defne children as patients the management of attention-defcit/hyperactivity 1 to 17 years old. Food and Drug Administration-Approved Indications and Dosages for Use in Pediatric Patients. However, not all of the medications in this drug class are approved for each indication. They are thought to work by increasing the neurotransmission of dopamine and norepinephrine. Short-acting stimulant medications may be easily titrated to dosages that produce symptom relief with manageable adverse reactions. They are often used as the initial treatment for children weighing less than 16 kg. Atomoxetine is not a controlled substance so it has a lower potential for substance abuse. Because of the cardiovascular use, however, both drugs also have related warnings about use in patients at risk for hypotension, heart block, bradycardia, syncope, other vascular diseases (including of the heart and brain), cardiac conduction abnormalities, and chronic renal failure. Establish a baseline for heart rate and blood pressure and monitor periodically, especially after initiation and dose increases. Both drugs also have warnings about somnolence and sedation, especially when used with a central nervous system depressant. Some of the treatment guidelines for the use of stimulant and related medications in pediatric patients are provided in Table 1. Treatment Guidelines for the Use of Stimulant and Related Medications in Pediatric Patients Sponsoring Organization Title of Guideline Link to Guideline National Institute for Health Attention defcit hyperactivity. The most common adverse reactions to stimulant and related medications are loss of appetite, upset stomach, insomnia, and headache. Other less common adverse effects include rebound irritability, dysphoria, agitation, tics, and growth impairment. Patients may experience increases in heart rate and blood pressure as a result of the sympathomimetic properties of stimulant medications. Patients should have a medical history and physical exam conducted prior to the initiation of therapy to assess cardiac disease, including family history of sudden cardiac death, family history of ventricular arrhythmia, or structural cardiac abnormalities. Patients with preexisting cardiac conditions should avoid the use of stimulant medications and the use of atomoxetine. The manufacturers of stimulant and related medications recommend a cardiac evaluation for any patient who presents with cardiac symptoms. Results showed no association between the use of stimulant medications and adverse cardiovascular events. The Medication Guides inform patients, parents, and caregivers about the possible cardiovascular risks and precautions that they may take to minimize the risks. The Medication Guides were recently updated to include information regarding circulatory problems. The Medication Guides have been revised to include this drug safety information as well. Events occurred most frequently in patients on established drug therapy undergoing dose escalation, but were also reported during withdrawal periods (drug holidays or discontinuation). The condition is not physical harmful, but the discoloration of skin may cause emotional distress in patients. If this occurs, the patient should not remove the patch until discussing an alternate therapy regimen with their provider. Rhabdomyolysis is the breakdown of muscle tissue, which is then released into the blood stream. Symptoms include dark, red, or cola-colored urine, decreased urine output, general weakness, and various muscle problems, including stiffness, aching, and tenderness. The previously mentioned Medication Guides also inform patients, parents, and caregivers about the risks of adverse psychiatric symptoms associated with stimulant and related medications. A follow-up study on methylphenidate suggests that children who are continuously treated with the medication experience a temporary decrease in growth rate without evidence of growth rebound during this period of development; however, there is inadequate data to determine whether chronic amphetamine use may cause similar suppression. The study recommends that growth should be monitored during treatment and to suspend treatment in patients who are not achieving expected growth or weight gain. Prescribing information warns of the high potential for abuse and also warns that extended use may lead to drug dependence. Administration of amphetamines for prolonged periods of time may lead to drug dependence. Misuse of amphetamine may cause sudden death and serious cardiovascular adverse reactions. The boxed warning for methylphenidate and methylphenidate derivatives is similar to the boxed warning for amphetamines. However, it informs providers to use caution when prescribing methylphenidate to patients with a history of drug dependence or alcoholism. The boxed warnings for methylphenidate medications are very similar to each other. Chronic abusive use can lead to marked tolerance and psychological dependence with varying degrees of abnormal behavior. Careful supervision is required during withdrawal from abusive use since severe depression may occur. Withdrawal following chronic therapeutic use may unmask symptoms of the underlying disorder that may require follow-up. Hepatotoxicity with Atomoxetine Atomoxetine has been shown to cause severe liver injury manifested by signifcantly elevated bilirubin concentrations and hepatic enzymes. Generic glimepiride 3 mg without a prescription. O que é Diabetes? Dr. Drauzio Varella.
There is still a lack of information among users diabetes test options purchase glimepiride with visa, particularly among 15 young people regarding the safety of solariums diabete facts buy glimepiride with american express. In Denmark diabetes prevention trial type 1 1 mg glimepiride with visa, not only the prevalence of sunbed 20 use in children is noticeable (Krarup et al diabetes numbers chart buy cheap glimepiride 4 mg on-line. During the action, tanning salons and similar facilities 9 were inspected, as well as the sunbeds offered there for use to the general public. The 10 overall conclusions from the results of the inspections in this action on sunbeds is that 11 consumer guidance in tanning studios is not regularly given and, where it is claimed to 12 be given this is often not verifiable. Moreover, the labelling of the sunbeds fails to 13 comply in at least 20% of the cases. In addition, how often the maximum values for 14 sunbeds are violated varies between the Member States. In several Member States the 15 percentage may be above 90%, while in others the percentage of sunbeds not complying 16 is estimated to be between 10% fi 20%. Compliance (solaria 40 and facilities) with national regulations and the effect of inspections delegated to local 12 europa. In 2008, 78 tanning facilities were selected 2 from six regions throughout Norway that contained municipalities with and without local 3 inspections. By 14 comparison, mean short and long wave irradiances of the inspected tanning devices in 15 2003 were 1. Local inspections gave better compliance with regulations, but 21 irradiances were significantly higher in municipalities with inspections (p fi 0. While according to the European fi 32 standard, erythemal-effective irradiance should not exceed 0. Application of a skin 35 cancer weighting factor, to compare the carcinogenic potential of sunbeds with that of fi 36 sunlight, produced values that varied from 0. In addition, the skin 40 cancer risk for comparable times of exposure was up to six times higher than that for 41 Mediterranean sunlight. Prevalence of sunbed use for tanning purpose is higher in white-skinned 15 populations from Northern Europe, and in young or middle-aged women. A recent meta16 analysis of data from 16 Western countries including 406,696 participants showed that 17 the overall summary prevalence of ever exposure to indoor tanning was as high as 18 35. This meta-analysis further showed an increase in prevalence of sunbed use 22 over time. There are few data on home use of sunbeds and 31 there is concern about uncontrolled use. Excessive exposure 5 results in signs of premature skin aging and the development of wrinkles. Long-term eye 6 damage including the formation of cataracts can also occur, as can eye irritation, photo7 keratitis and conjunctivitis. Melanoma is 10 fast growing, proliferates readily and is lethal unless detected early. In addition, vitamin D plays a role in cell 27 growth and in reducing inflammation; the function of many genes is modulated by 28 vitamin D, and many cells have vitamin D receptors (Holick 2007, Fleet 2012). Recent reviews have re-examined the 33 association of low vitamin D status with cancer and with mortality (Yin 2013, Autier 34 2014, Schottker 2014). These analyses confirm the association with colon cancer, 35 whereas the association with other types of cancer is as yet unclear. A systematic review 36 supports the notion that low vitamin D status is often a consequence of (chronic) 37 inflammatory disease (Autier 2014). Its optimal level in the blood is not known, but levels below 10ng/ml are 40 considered to indicate deficiency. Further conversion into the physiologically active 2543 hydroxyand 25-dihydroxy-vitamin D occurs in the liver and kidney. Extensive sunbed 16 exposure may therefore undermine vitamin D production (Levine 2005). It must be 17 noted that other sources of adequate vitamin D supply to the human body are available. When this is 19 impractical, or impossible, then dietary sources (especially fish) or vitamin D 20 supplements (10 microgram/day) are suitable and affordable alternatives. Chronic low 21 vitamin D status is a medical issue for which treatment by means of diet, supplements or 22 (in rare cases) medication is the best possible approach (Diffey 2011). It is a gradual process, which is irreversible, even if the low-level 30 inflammation is reversed. This phenomenon has been reproduced in skin 36 samples taken from volunteers who started to use sunbeds (Reimann 2008). Feelings 46 like being comfortable and the perceived cosmetic attractiveness of a tanned skin are 33 1 reported by sunbed users (Brandberg 1998, Bloodstock 1992), although having a tan is 2 not an issue in several cultures. However, the criteria to assess the prevalence of 10 tanning dependency have been challenged (Schneider 2015). The human studies on plasma beta-endorphin have thus far not 13 demonstrated clear evidence of raised blood levels (Kaur 2006b). Based on 40 19 informative published studies (18 case-controls, of which 9 were population based, 41 and one cohort) that included 7 355 melanoma cases and 11,275 controls from case42 control studies and 106,378 cohort members. In 17 fact Grant is mistaken in that 8 of the 19 studies included in the meta-analysis were 18 adjusted for multiple confounders. The authors suggest that this result implies 38 that newer tanning technology is not safer than the older one. Among potential participants, 16 1167 case patients and 1101 control subjects (84. Adjustment was made for potential confounders including age, gender, eye 20 and skin colour, freckles and moles, annual income, education, family history of 21 melanoma, lifetime sun exposure (routine, leisure activities outdoors, during work) and 22 sunscreen use. These factors 43 were adjusted for in multivariate analyses by Lazovich et al; Grant et al suggest 44 additional analyses stratified by these factors would be informative. They suggest that sunbeds 13 have an effect on melanoma independently from the effect of sunburns and that the 14 additive effect could have been masked by using models that assume a multiplicative 15 effect. Among those who had ever used a sunbed and were 33 diagnosed between 18 and 29 years of age, three quarters (76%) of melanomas were 34 attributable to sunbed use. The locations where 38 sunbeds were used were private home (54%), tanning salons (34%), gyms/spas (32%), 39 hairdressers/beauty salons (13%) and hospital/medical facilities (9%). Number of 45 sessions and years since first use did not show an increasing trend effect on melanoma 46 risk. They point out that having 44% fewer controls than cases is an 3 unusual feature of a case-control study, and that the family doctors who selected 4 controls did not appear to have successfully selected controls who were within 5 years of 5 age of the cases as a large imbalance in age of cases and controls resulted; controls 6 were also of a higher socioeconomic status than the cases. They also suggest that the 7 use of sibling controls may be problematical in that siblings may share identical 8 behaviours such as visiting indoor tanning parlours. For females, the individual risk for melanoma increased with 29 typical session time and frequency of sessions. Body sites that are not generally 34 exposed to sunlight were more common sites of primary melanomas for frequent sunbed 35 fi sunlamp users. For males, measures of sunbed fi sunlamp use were not significantly 36 associated with melanoma risk. Exposure data, 40 including sunlamp and sunbeduse, were collected by telephone interview. About 17% of 41 participants had used a sunlamp at least once and most use (89%) occurred before 42 1980. The overall prevalence of sunbed use was 22% (86 cases and 102 47 controls) and most use (83%) occurred after 1980. The 4 authors suggest that there a sufficient lag time may not have elapsed to assess a true 5 effect. Most have a large sample size and collect and adjust for relevant confounders 10 such as sunlight exposure, hair colour, presence of moles/freckles etc. It should be 11 noted that the use of sunbeds was generally self-reported and there was generally no 12 information on the specific sunbed type used. However, this study was 15 unusual in design in that there were fewer controls than cases, there was an imbalance 16 of age between cases and controls and some of the controls were case siblings for whom 17 there may have been similar behaviours. A new analysis of the Norwegian27 Swedish cohort and two new cohorts are described below. This study was conducted in Norway 31 and Sweden and included 106,379 women aged 30 to 50 years at recruitment in 199132 1992.
Single-use carton 1 vial of medication diabetes insipidus urinalysis purchase glimepiride visa, 1 prefilled diluent syringe (containing 1 diabetes diet best fruits cheap glimepiride online visa. Interferon beta-1b is manufactured by bacterial fermentation of a strain of Escherichia coli that bears a genetically engineered plasmid containing the gene for human interferon betaser17 diabetes diet uptodate cheap 1 mg glimepiride. The native gene was obtained from human fibroblasts and altered in a way that substitutes serine for the cysteine residue found at position 17 diabetes test buy cheap glimepiride. Interferon beta-1b is a highly purified protein that has 165 amino acids and an approximate molecular weight of 18,500 daltons. An exacerbation was defined, per protocol, as the appearance of a new clinical sign/symptom or the clinical worsening of a previous sign/symptom (one that had been stable for at least 30 days) that persisted for a minimum of 24 hours. Patients selected for study were randomized to treatment with either placebo (n = 123), 0. Patients who required more than three 28-day courses of corticosteroids were withdrawn from the study. A number of secondary outcome measures were also employed as described in Table 8. Results at the protocol designated endpoint of 2 years (see Table 8): In the 2-year analysis, there was a 31% reduction in annual exacerbation rate, from 1. Of the first 372 patients randomized, 72 (19%) failed to complete 2 full years on their assigned treatments. Figure 1 displays a histogram of the proportions of patients who fell into each of these intervals. The percentage of scans with new or expanding lesions was 29% in the placebo group and 6% in the 0. At the end of 2 years on assigned treatment, patients in the study had the option of continuing on treatment under blinded conditions. As noted above, in the 2-year analysis, there was a 31% reduction in exacerbation rate in the 0. In the analysis of the third year alone, the difference between treatment groups was 28%. The lower number of patients may account for the loss of statistical significance, and lack of direct comparability among the patient groups in this extension study make the interpretation of these results difficult. Throughout the clinical trial, serum samples from patients were monitored for the development of antibodies to interferon beta-1b. One-third had neutralizing activity confirmed by at least 2 consecutive positive titers. This development of neutralizing activity may be associated with a reduction in clinical efficacy, although the exact relationship between antibody formation and therapeutic efficacy is not yet known. Disease had to be in the secondaryprogressive phase and deterioration could not be exclusively related to incomplete recovery from relapses. The increased score had to be maintained for 3 months before progression was confirmed. Although the study was designed with a treatment duration of 3 years, a prospectively planned interim analysis of efficacy was performed after all patients had completed 2 years in the study. This resulted in a decision by an independent Advisory Board to terminate the study early. The primary analysis of efficacy was based on all patients randomized to treatment (intent-to-treat). All efforts were undertaken to maintain the blinding, eg, standard clothing to cover injection sites was obligatory. In both treatment groups, the proportion of female patients exceeded that of males (placebo: 64. Eighty-eight (88) patients were lost to follow-up; the remainder was followed up until the end of study irrespective of continuation of study drug. Lack of efficacy, adverse events and noncompliance were the most common reasons for ending treatment in 15. The delay in progression in disability became apparent after 9 months of treatment and was statistically significant from month 12 onwards. The proportion of patients with confirmed progression in disability was reduced from 60. When patients lost to follow-up were assumed not to have progressed, the respective values were 16. The Kaplan-Meier estimate of the percentage of patients progressing by the end of 3 years was 53. Figure 2: Onset of Progression in Disability by Time in Study (Kaplan-Meier Methodology: Posthoc Analysis) 3. Patients with monofocal or multifocal onset of the disease were included (ie, patients with clinical evidence of a single or at least 2 lesions, respectively, of the central nervous system). Any disease other than multiple sclerosis that could better explain the signs and symptoms of the patient had to be excluded. This study consisted of 2 phases, a placebo-controlled phase followed by a pre-planned follow-up phase. Multiple sclerosis according to the McDonald criteria was reached if, in addition to the single clinical demyelinating event, both dissemination in space and dissemination in time had been established. A posthoc analysis adjusting for the standard baseline covariates of steroid use during single event, type of disease onset (multifocal versus monofocal), age, sex, number of T2 lesions, and number of gadolinium-enhancing lesions, revealed a similar risk reduction of 50%. The results of subgroup analyses should be interpreted with caution since the clinical trial was not designed to evaluate efficacy in subgroups. T2-lesion volumes decreased from screening to the end of study in the majority of patients in both treatment groups due to regression of inflammatory changes that had been associated with the first clinical event. All patients who completed the placebo-controlled phase according to the protocol were eligible to enter the follow-up phase and, after renewing their written informed consent, were offered 0. Throughout the follow-up phase, patients, investigators, and raters were kept blinded to the original treatment allocation from the placebo-controlled trial. Two preplanned analyses of the integrated data after completion of years 3 and 5 were performed. By the fifth year, the majority of patients had been treated for at least 3 years. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. Application of the new McDonald criteria to patients with clinically isolated syndromes suggestive of multiple sclerosis. Human biologic response modification by interferon in the absence of measurable serum concentrations: a comparative trial of subcutaneous and intravenous interferon-beta serine. Systemic recombinant human interferon-beta treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up. Lack of association between antimyelin antibodies and progression to multiple sclerosis. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Binding of recombinant-produced interferon beta ser to human lymphoblastoid cells. Effects of combinations of interferon-beta ser and interferon-gamma on interferon-inducible proteins and on the cell cycle. Discrepancies in the interpretation of clinical symptoms and signs in the diagnosis of multiple sclerosis. Pharmacodynamics of biological response in vivo after single and multiple doses of interferon-beta. Diluent: sodium chloride, water for injection Contact your doctor or pharmacist if you have any questions about the drug. This test has to fi Heart problems show at least two signs of inflammation in the central nervous fi Problems with your thyroid gland system suggestive of multiple sclerosis. Less to play an important part in the process which damages the severe allergic reactions such as rash, itching, skin bumps, or nervous system. |