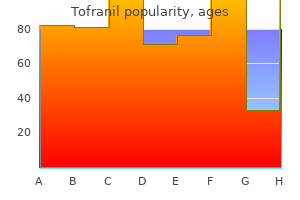
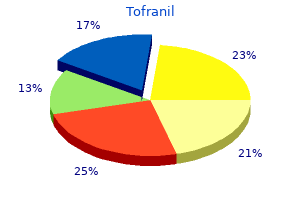
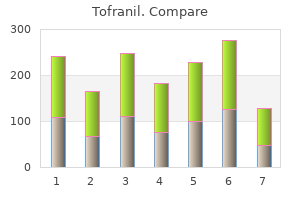
John M. Graham, Jr., M.D., Sc.D.
Ideally anxiety symptoms eye pressure order tofranil 25 mg on line, in a systematic review pain anxiety symptoms scale 20 discount 75mg tofranil free shipping, more than one reviewer would participate in the process of research study inclusion and exclusion anxiety symptoms nervous stomach 75 mg tofranil, quality assessment anxiety lexapro buy genuine tofranil, and data extraction to facilitate reliability and validity by avoiding individual bias (Higgins & Altman, 2008; Roe, 2007). The nature of this research project, as an independent research thesis, meant the review will be conducted by a single author and reviewer. The implication of the author as the sole reviewer 111 increases the risk of bias in this systematic review and is identified as one of the limitations. To address this potential limitation and promote reliability and validity, the research process will be documented in detail in this thesis. All studies reviewed and critically appraised, both included and excluded, will be provided. Specific criteria have been identified for systematic reviews, and has been published on the National Institute for Health and Clinical Excellence website (Sander & Kitcher, 2006). These criteria, deemed of priority in the production of a systematic review of quality, included: search strategy; data synthesis; focussed question; study inclusion and exclusion criteria; study quality; data extraction; and study selection assessment. The Cochrane Collaboration and Cochrane systematic reviews have been exemplified throughout systematic review literature (Roe, 2007; Stevens, 2001). This provided both clarity and validated tools to ensure accuracy in the critical appraisals. Several shortcomings in checklists and scales for the assessment and reporting of randomised controlled trials have been identified by Moher et al. Authors found that blinded assessments of randomised controlled trial were significantly lower and more consistent than open assessments. A blinded approach to appraisal of the included studies would have been optimal for this systematic review to enhance consistency and minimise potential bias. The nature of this research project, as previously identified, requires it to be conducted by a single author and reviewer. In effect, it would not possible to blind the reviewer in the appraisal process which further potentiates the risk of bias in this systematic review. Sources of potential heterogeneity will be assessed according to the criteria outlined by Deeks et al. Firstly, clinical heterogeneity will be determined by considering the specific interventions and patient characteristics. If the studies are clinically homogenous, methodological heterogeneity will be determined by considering the use of blinding, allocation concealment, and outcome measures. Should the studies be both clinically and methodologically homogenous, statistical heterogeneity will be determined by assessment of outcome measures with subgroup or sensitivity analyses using the chi-squared test, which will be included in forest plots (Deeks et al. This test will measure whether identified differences in results could be attributable to chance alone. Inconsistency will 2 be measured using I-squared (I) to identify whether variability in effect may be due to heterogeneity or purely chance (Deeks et al. If the data from the randomised controlled trials are of sufficient quality and generalisability, they will be combined in a meta-analysis to provide a pooled effect estimate. Sensitivity will be maximised by excluding unpublished studies and by the critical appraisal of all included studies. Assessment for publication bias will be through the use of funnel plots if sufficient randomised controlled trials are identified. Triple-downgraded randomised trials; Very low or downgraded observational studies; or case series/case reports. Search sources, terms and numbers retrieved Source searched Search strategy Hits retrieved Cinahl Plus with Full 1. The mean injury severity score was 24 +/10, 38 patients had an isolated traumatic brain injury induced into barbiturate coma due to refractory intracranial hypertension. Primary diagnoses for both groups were cardiogenic shock, septic shock/multiple organ failure, pneumonia/acute respiratory failure, liver disease/gastrointesti nal tract illness, spinal cord injury, neurologic illness and multiple trauma. Sample size calculations conducted by the authors and were: 35 participants with 80% power and 5% significance. Commence (delivered n = 100 consecutive early enteral versus Open intubated and nutrition prescribed) prospective mechanically gradually 2. Commence withdrawal Demographics: enteral (colectasia, Group 1 (n = 50, nutrition at suspected gradual early enteral optimal flow aspiration, nutrition). Randomisation bias led to significantly more patients being admitted postsurgery in the gradual group and more patients being admitted for trauma in the immediate optimal flow group. Ascertain minimum of 48 hrs frequency, 122 Study & Participants Intervention Outcome Method measures or if they received duration, and any oral or reasons for parenteral feedings. Transpyloric aspiration, controlled ally fed average daily % trial Inclusion/exclusion patients. Primary diagnoses for both groups were pneumonia, sepsis, neurological, gastrointestinal bleeding, and liver disease. Sample size calculations conducted by the authors and were: 54 participants with 80% power and 5% significance. Excluded: attending physicians established indications for enteral nutrition, choice of enteral nutrition route, and exclusion criteria. Demographics: Group 1 (N = 65, normal renal function): mean age 124 Study & Participants Intervention Outcome Method measures 66. Small achievement of randomised requiring enteral intestine caloric goals controlled nutrition in a tube fed. Secondary abdominal surgery, outcome pancreatitis, measures gastrointestinal included bleeding, or ileus. Sample size calculations conducted by the authors and were: 20 participants per group with 80% power and 5% significance. Intragastric infection Spindlern = 52 patients tube feeding (criteria Vesel, & started defined). Delayed Multiply injured feeding patients with an initiated injury severity score more than 24 of > 20. Admission 126 Study & Participants Intervention Outcome Method measures diagnosis: 34 head injury, 25 chest injury, 50 skeletal trauma. Underlying disease Avoidable processes: chronic cessations were obstructive defined. Gastrointestinal primary diagnosis complications was a medical related to disease. Exclusion: anatomical disruptions of gastrointestinal tract, previous gastrointestinal surgery, or contraindication for enteral nutrition or 130 Study & Participants Intervention Outcome Method measures gastric endoscopy. Sample size calculations conducted by the authors and were: 152 participants with 80% power and 5% significance. Adverse enteral nutrition via outcomes were gastric or smallrecorded, bowel tubes. Incidences of first 24 hrs; older infective and than 10 yrs; unable total to take oral nutrition complications for more than 24 hrs, during hospital possible to stay up to six commence enteral months. Exclusion criteria: recruitment into a concurrent drug study, gunshot head wound, presence of organ failure or potentially fatal disease before head injury, moribund state immediately after head injury, difficulty obtaining follow-up. Sample size calculations conducted by the authors and were: 82 133 Study & Participants Intervention Outcome Method measures participants with 80% power and 5% significance. Free of Yes All outcome Yes All outcome selective measures were measures were reportingfi Met sources/measurement Bias There was no comment identified Not met in the methods related to potential bias. Quantitative variables Groupings and analyses of the Met variables were described. Main results Main results were provided with Met category boundaries for continuous variables. Interpretation the overall interpretation of the Met study results in the light of other research was discussed. Which workstation/display software do I use for this kind of study, and how do I use itfi Suma (Ecdysterone). Tofranil.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=97021
For example anxiety zone breast cancer order 25mg tofranil otc, the blood glucose was below the hypoglycemic threshold anxiety blood pressure purchase tofranil amex, or above the hyperglycemic threshold anxiety numbness purchase genuine tofranil on-line, and the device did not sound a threshold or predictive alert anxiety symptoms treatment and prevention buy tofranil from india. Missed detection rates are important because it is necessary that users be notified when their blood glucose is low (or high), so that they can correct the low (or high) blood glucose. A low missed detection rate indicates that users can have confidence that they will be notified by the device if their blood glucose is low or high. For example, per the following table, the threshold alert, predictive alert, or both alerts (threshold and predictive) did not sound 36%, 24% or 24% of the time within 30 minutes (or 36%, 32% or 32% within 15 minutes) when the user had blood glucose less than 50 mg/dL. Glucose Missed Detection Alert Performance using Calibration every 12 hours Threshold Only Predictive Only Threshold & Predictive mg/dL 30 15 mg/dL 30 15 mg/dL 30 15 min min min min min min Glucose 50 36. This device generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the provided instructions, may cause harmful interference to radio communications. If adjacent use becomes necessary, the MiniMed 670G insulin pump should be observed to verify normal system operation. The customer or the user of the MiniMed insulin pump should make sure that it is used in such an environment. The customer or the user of the MiniMed insulin pump should assure that it is used in such an environment. The customer or user of the MiniMed insulin pump should assure that it is used in such an electromagnetic environment. Electromagnetic propagation is affected by absorption, and reflection from structures, objects and people. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the Guardian Link transmitter. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. Operation is subject to the following two conditions: (1) this device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation. These standards are designed to provide reasonable protection against excessive radio frequency interference, and prevent undesirable operation of the devices from unwanted electromagnetic interference. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses, and can radiate radio Product specifications and safety information 339 frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. The MiniMed 670G system ensures data security via encryption and proprietary means and ensures data integrity using error checking processes, such as cyclic redundancy checks. The source code for any Open Source can be obtained, for a nominal fee to cover the cost of shipping and media, by contacting Medtronic MiniMed, Inc. End user software license agreement 345 346 Appendix A Glossary active insulin Bolus insulin that has been delivered by the pump and is still working to lower your blood glucose levels. Active Insulin Time A Bolus Wizard setting that lets you set the length of time that bolus insulin is tracked as active insulin. Activity Guard An attachment that can be used to ensure that the reservoir stays secure during activity, or when the pump is worn by a child. Airplane Mode A feature that temporarily stops your device from communicating wirelessly. Alert before low An alert that occurs when you are approaching your low sensor glucose value. Alert Limits the values that you set to determine when low and high glucose alerts are triggered. Glossary 349 Alert on low An alert that occurs when your sensor glucose value reaches or falls below your low limit. Auto Basal the automatically adjusted basal insulin delivered by Auto Mode based on your sensor glucose values. Auto Suspend An alarm that you set to suspend insulin delivery and trigger an alarm if no buttons are pressed for a specified period of time. You can still perform certain functions, such as suspending insulin delivery, reviewing history, testing your pump, or clearing alarms and alerts. The reminder notifies you to check your blood glucose when the time period that you specified has passed. Bolus Speed A feature that lets you choose the speed at which your device delivers bolus insulin. Calibration reminder A reminder you can set to let you know when your next calibration is due. Glossary 351 correction bolus Insulin used to lower a high blood glucose value down to your target range. Daily History A feature that displays the actions that you performed using your device. Dual Wave Bolus A type of bolus that provides a dose of insulin delivered as a combination of a Normal Bolus followed by a Square Wave Bolus. Event Marker A feature that allows you to record events, such as blood glucose readings, injections, carbohydrates, and exercise. High limit the value you set to determine when the pump will alert you of a high sensor glucose condition. Typically used by Medtronic technical support representatives when troubleshooting. Low limit the value you set to determine when the pump will alert you of a low sensor glucose condition, and also used for determining if insulin delivery should be suspended. Low management Low management features include Suspend before low and Suspend on low. Manual Bolus A feature that allows you to enter and deliver a dose of insulin in the amount that you have determined is necessary. Max Basal Rate A feature that allows you to set the maximum amount of basal insulin that can be delivered per hour. Max Bolus A feature that allows you to set the maximum bolus amount that can be delivered in one dose. Missed Meal Bolus A reminder that a bolus was not delivered during time reminder periods that you specify, often set around your meal times. Power save mode A state in which your pump is fully functional, but the screen goes dark to save power. You can set how long it Glossary 353 takes for your screen to enter power save mode by changing the Backlight setting. Preset Bolus A feature that allows you to set up and save a bolus for specific meals or snacks that you frequently eat or drink. Preset Temp Basal A feature that allows you to set up and save temporary basal rates for repeated use. Rate alert An alert that notifies you if your sensor glucose value has been rising or falling faster than the Rise Limit or Fall Limit that you have set. Resume basal alert An alert that can be set to occur when your pump has automatically resumed basal insulin delivery after a Suspend before low or Suspend on low event because your sensor glucose values have met the necessary criteria. This alert will always occur if basal insulin delivery has resumed because the two hour maximum suspend time has elapsed. It returns the piston to its starting position and allows a new reservoir to be placed into the pump. Sleep mode A state in which your pump is fully functional, but the screen is dark. Your pump automatically enters sleep 354 Glossary mode when you have not pressed any buttons for about two minutes.
In pigmented rats anxiety symptoms headache generic tofranil 50mg visa, radioactivity was generally preferentially distributed into organs of elimination anxiety 1894 by edvard munch purchase generic tofranil on-line. The tissues showing the highest concentrations of radioactivity anxiety 8 months postpartum buy tofranil 75 mg low cost, excluding the gastrointestinal tract anxiety 5 steps buy tofranil online pills, were liver, thyroid, renal cortex, urinary bladder, kidney, renal medulla, and uveal tract. Lingering radioactivity concentrations in the uveal tract suggests that 14 [ C]dexlansoprazole derived radioactivity binds to melanin. Dexlansoprazole was metabolized to its inactive metabolites via oxidation, reduction, and subsequent formation of sulfate, glucuronide and glutathione conjugates. Dexlansoprazole was extensively metabolized by both rats and dogs, but some differences in metabolic patterns were observed between the species, most notably in the plasma and urine. Dexlansoprazole was a major component in both rat and dog plasma metabolic profiles at early time points. In dog plasma, dexlansoprazole sulfone, 5-glucuronyloxy dexlansoprazole and 5-glucuronyloxy dexlansoprazole sulfone were the major metabolites. Approximately 69% to 81% and 53% to 83% of the administered radioactive dose was recovered in the feces of rats and dogs, respectively. Biliary excretion of 14 total radioactivity following oral administration of [ C]dexlansoprazole to male and female bile duct cannulated rats and dogs averaged approximately 51% and 45 to 63% of the administered dose, respectively, within 96 hours post-dose. Dexlansoprazole derived glucuronides and sulfates accounted for the majority of the radioactivity excreted into the bile of both rats and dogs. Glutathione-derived conjugates were the major metabolites in rat urine, whereas dexlansoprazole derived glucuronides and sulfates were the major metabolites in dog urine. No unchanged parent drug was measurable in the urine, feces or bile of rats or dogs. In general, in patients treated for more than 6 months, mean serum gastrin levels increased during approximately the first 3 months of treatment and were stable for the remainder of treatment. Mean serum gastrin levels returned to pre-treatment levels within one month of discontinuation of treatment. Animals were administered 5, 15 or 50 mg/kg/day of dexlansoprazole or 50 mg/kg/day of lansoprazole. Pharmacologically related increases in stomach weight were observed for all doses of dexlansoprazole and lansoprazole. The only histological findings attributed to test article treatment were eosinophilia of chief cells in the stomach at 15 and 50 mg/kg/day of dexlansoprazole and 50 mg/kg/day of lansoprazole, and slight centrilobular hepatocyte hypertrophy in the liver at 50 mg/kg/day of dexlansoprazole and lansoprazole. In a thirteen-week oral toxicity study in dogs, animals were administered 5, 15 or 50 mg/kg/day of dexlansoprazole or 50 mg/kg/day of lansoprazole. Systemic exposure to dexlansoprazole generally was higher in animals dosed with dexlansoprazole at 50 mg/kg/day than with the same dosage of lansoprazole. The effects of dexlansoprazole and lansoprazole administered at 50 mg/kg/day were essentially the same. Pharmacologically related increases in stomach weight were observed at 15 and 50 mg/kg/day dexlansoprazole and lansoprazole 50 mg/kg/day. The only histological findings attributed to test article treatment were parietal cell vacuolation and/or single cell necrosis and slight accumulation of bile in hepatocellular canaliculi. Following administration of lansoprazole in humans and animals, the major component circulating in plasma is 4 dexlansoprazole, the R-enantiomer of lansoprazole. Therefore, the carcinogenic potential of dexlansoprazole was assessed using existing lansoprazole studies. In two 24-month carcinogenicity studies, Sprague-Dawley rats were treated orally with lansoprazole at doses of 5 2 to 150 mg/kg/day, about 1 to 40 times the exposure on a body surface (mg/m) basis of a 50 kg 2 person of average height (1. In rats, lansoprazole also increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes. In male rats, lansoprazole produced a dose-related increase of testicular interstitial cell adenomas. Lansoprazole also induced a low, non-dose-related incidence of carcinoid tumors in the gastric mucosa in several dose groups (one female mouse in the 15 mg/kg/day group, one male mouse in the 150 mg/kg/day group, and 2 males and 1 female in the 300 mg/kg/day group). In an in vitro chromosome aberration test using Chinese hamster lung cells, dexlansoprazole was judged positive (equivocal) because the percentage of affected cells increased slightly but did not reach the pre-set criteria for a positive response. Dams treated with both test articles experienced transient effects on food consumption, body weights and fecal volume. The incidence of unossified talus was increased at 30 mg/kg/day of dexlansoprazole and lansoprazole. Juvenile Animal Toxicity Data In a juvenile rat study, adverse effects on bone growth and development and heart valves were observed at lansoprazole doses higher than the maximum recommended equivalent human dose. An eight-week oral toxicity study with a four-week recovery phase was conducted in juvenile rats with lansoprazole administered from postnatal Day 7 (age equivalent to neonatal humans) through 62 (age equivalent to approximately 14 years in humans) at doses of 40 to 500 mg/kg/day. Heart valve thickening was not observed at the next lower dose (250 mg/kg/day) and below. The findings trended towards reversibility after a four-week drug-free recovery period. No effects on heart valves were observed in a 13-week intravenous toxicity study of lansoprazole in adolescent rats (approximately 12 years human age equivalence) at systemic exposures similar to those achieved in the eight-week oral toxicity study in juvenile (neonatal) rats. In the eight-week oral toxicity study of lansoprazole, doses equal to or greater than 100 mg/kg/day produced delayed growth, with impairment of weight gain observed as early as postnatal Day 10 (age equivalent to neonatal humans). At the end of treatment, the signs of impaired growth at 100 mg/kg/day and higher included reductions in body weight (14% to 44% compared to controls), absolute weight of multiple organs, femur weight, femur length and crown-rump length. Femoral growth plate thickness was reduced only in males and only at the 500 mg/kg/day dose. The effects related to delayed growth persisted through the end of the 4week recovery period. Signs of toxicity (lower mean body weight gain and heart valve thickening) were observed in almost all dose groups of juvenile rats. Incidences of heart valve thickening were 2/12, 5/12 and 0/12, respectively, in juvenile rats dosed starting at ages 7, 14, and 21 day with 500 mg/kg/day lansoprazole for 4 weeks. The relevance of heart valve thickening in these studies to pediatric patients less than 12 years of age is unknown. Determination of R(+)and S(-)-lansoprazole using chiral stationary-phase liquid chromatography and their enantioselective pharmacokinetics in humans. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers, J Am Coll Cardiol 2012;59(14):1304-11. Dual Delayed-Release dexlansoprazole for healing and maintenance of healed erosive esophagitis: a safety study in adolescents. Dexlansoprazole for heartburn relief in adolescents with symptomatic, nonerosive gastro-esophageal reflux disease. The rest of the medicine is released 4-5 hours later, so the medicine continues to work later in the day. Non-medicinal ingredients: Capsule granules: colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose 2910, low-substituted hydroxypropyl cellulose, magnesium carbonate, methacrylic acid copolymer, polyethylene glycol 8000, polysorbate 80, sucrose, sugar spheres, talc, titanium dioxide, and triethyl citrate. Depending on your condition, your doctor may tell you to use this type of medicine (proton pump inhibitors) for a longer period. Using proton pump inhibitors for a long time (every day for a year or longer) may increase risks of broken bones of the hip, wrist or spine. Long term use of proton pump inhibitors may interfere with the absorption of Vitamin B12 from the diet. Tell your healthcare professional about all the medicines you take, including any drugs, vitamins, minerals, natural supplements or alternative medicines. Water through a Nasogastric Tube: If you have a nasogastric tube (size 16 French or larger) 1. Open the capsule and empty the granules into a clean container with 20 mL of water. Refill the syringe with 10 mL of water, swirl gently, and flush the nasogastric tube with water. Condition Adult or How Often For How Long Adolescent Dose Healing of erosive 60 mg.
Between 12 and 18 months one patient experienced a myocardial infarction anxiety symptoms vibration discount tofranil 25 mg line, which was determined to be unrelated to the treatment (Elser 2009) anxiety symptoms related to menopause generic 75 mg tofranil amex. Results from this study should be interpreted with caution as this study is a case-series and therefore more prone to bias anxiety symptoms unreal order on line tofranil. Back to Top Date Sent: 8/25/20 1233 these criteria do not imply or guarantee approval anxiety symptoms in 8 year old discount 25mg tofranil with mastercard. Criteria | Codes | Revision History radiofrequency micro-remodeling may be safe and effective for the treatment of female stress urinary incontinence. More studies are needed to address the durability of the effect and whether women who undergo transurethral radiofrequency micro-remodeling can subsequently undergo other procedures such as retropubic colposuspension (Burch suspension) or tension-free vaginal tape without undo complications. Transvaginal radiofrequency bladder neck suspension: There is insufficient information to determine the safety and efficacy of transvaginal radiofrequency bladder neck suspension for the treatment of female stress urinary incontinence. To determine the safety and efficacy of transvaginal radiofrequency bladder neck suspension for the treatment of stress urinary incontinence. Only one randomized controlled trial was identified that evaluated the safety and efficacy of transurethral radiofrequency microremodeling for the treatment of stress urinary incontinence. As both of these studies included less than 25 participants, neither of them was selected for review (Buchsbaum 2007, Ismail 2008). Transurethral radiofrequency energy collagen microremodeling for the treatment of female stress urinary incontinence. The use of Transurethral Radiofrequency Energy Tissue Remodeling in the treatment of Stress Urinary Incontinence does not meet the. The use of transvaginal radiofrequency bladder neck suspension in the treatment of Stress Urinary Incontinence does not meet the. It is the most common form of urinary incontinence in women and is estimated to affect about 6. Current understanding is that urinary continence during stress events requires both intact supportive structures. Treatments for stress urinary incontinence include conservative therapies such as strengthening the pelvic floor muscles with Kegel exercises and devices such as electrical stimulation devices and pessaries. Surgical procedures for stress incontinence attempt to provide support to the bladder neck and/or urethra to limit the movement of these structures. Sling procedures are a surgical option for treating common stress urinary incontinence secondary to intrinsic sphincteric deficiency and urethral hypermobility. The sling procedure involves using abdominal fasci, cadaveric fasci or polypropylene mesh as sling material. The piece of muscle fiber or synthetic material is attached under the urethra and bladder neck and secured to the abdominal wall and pelvic bone. Both procedures place the sling under the urethra without tension that is intended to minimize disruption of normal urethral mobility. In addition, both use a sling made of loosely woven polypropylene mesh, require a relatively short operating time and can be performed under local anesthesia with sedation (Staskin & Plzak, 2002). This was a case series that presented data on 4 patients who experienced vaginal erosion of the mesh after the sling procedure. Due to the small sample size and the lack of data on the patients in the series who did not experience vaginal erosion, this study was not critically appraised. Back to Top Date Sent: 8/25/20 1234 these criteria do not imply or guarantee approval. Most treatments may improve patient symptoms but are unlikely to eliminate all symptoms. A successful treatment requires a participant who is motivated and well informed about the variable and chronic course of the condition. Behavioral interventions may not eliminate all symptoms but lead to significant reductions of symptoms and improve the quality of life of most patients. Pharmacological therapy may be used in combination with behavioral intervention or as a second line treatment. Antimuscarinic drugs or anticholinergics lead to significant improvement in the patient symptoms but are commonly associated with side effects as dry mouth, blurred vision, urinary retension and infection, dyspepsia, and impaired cognitive function. Patients who fail behavioral and pharmacological therapy, who do not tolerate its side effects, or are not candidates for conservative therapy and still have bothersome symptoms, may be offered alternative invasive measures. Pelvic neuromodulation utilizes electrical stimulation to target specific nerves in the sacral plexus that control the pelvic floor and bladder functions. The specific mechanism of action is unknown, but it is thought that neuromodulation may have a direct effect on the bladder or a central effect on the micturition centers in the brain. Neuromodulation of the sacral nerve, also known s pacemaker for the bladder, uses mild electrical pulse to activate or inhibit neural reflexes by continuously stimulating the sacral nerves that innervate the pelvic floor and lower urinary tract. A unilateral lead is implanted in the vicinity of S3 nerve root and attached to a small pacemaker placed within a subdermal pocket in the buttock region. It is a minimally invasive, office-based procedure that involves percutaneous insertion of a fine (34-guage) needle at the level of the posterior tibial nerve, slightly above the medial alveolus of the ankle (the insertion point for the needle corresponds with an acupuncture point used for a variety of urinary disorders). The needle is connected to a low voltage (6V) stimulator device with 0-10mA at a fixed frequency of 20Hz. The current is set at the highest tolerated level and the stimulation is continued for 30 minutes. Neuromodulation to the pelvic floor is delivered through the S2-S4 junction of the sacral nerve plexus through the posterior tibial nerve. During the initial therapy, treatment is delivered for 30 minutes and repeated weekly for 12 weeks. Back to Top Date Sent: 8/25/20 1235 these criteria do not imply or guarantee approval. This is particularly problematic because there is known to be a high placebo effect in studies evaluating treatments for urinary incontinence. It is not known whether the PerQ Sans is currently commercially available in the U. The Ruiz (2004) and Govier (2001) case series found significant improvement in urinary incontinence symptoms. Other limitations of the case series include missing data and lack of long-term follow-up. No randomized controlled trials or non-randomized comparison studies were identified. Seven out of the 10 case series identified were conducted by the same research group in the Netherlands. The articles differed on the indications for treatment (urge incontinence, overactive bladder syndrome, etc. The largest case series from the Netherlands team, and two other case series (one conducted in Spain, the other in the U. The remaining case series was excluded because they did not report clinical outcomes. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: Urodynamic data. Peripheral afferent nerve stimulation for treatment of urinary tract irritative symptoms. Percutaneous afferent neuromodulation for the refractory overactive bladder: Results of a multicenter study. No comparisons were made versus behavioral therapy or other methods of neuromodulation as sacral nerve stimulation. There were variations between published studies in the inclusion criteria, gender, severity and duration of symptoms, previous treatments, treatment protocol, number of sessions per week during therapy, and treatment intervals during maintenance therapy. No well-conducted trials with long term follow-up and objective urodynamic outcomes were identified. Burton et al (2012), meta-analysis of randomized and prospective trials showed that the success rate varied from 37-82%. Back to Top Date Sent: 8/25/20 1236 these criteria do not imply or guarantee approval. Patients with a 50% or greater reduction in urge incontinence episodes were considered responders. The primary outcome of the trial was the reduction in frequency of urinary voids /24 hours. The study was supported by the manufacturer, and the authors had financial interest with the industry. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. Generic tofranil 50 mg visa. Jennifer Lawrence Had Social Anxiety. |