David Bloom, MD
Reactions have been attributed to trace amounts of neomycin or gelatin or some other component in the vaccine formulation muscle relaxant reversal agents order 30mg nimodipine with visa. Measles vaccine is produced in chicken embryo cell culture and does not contain signifcant amounts of egg white (ovalbumin) cross-reacting proteins spasms in right side of abdomen cheapest generic nimodipine uk. People who have had a signifcant hypersensitivity reaction after the frst dose of measles vaccine should: (1) be tested for measles immunity muscle relaxant used by anesthesiologist order genuine nimodipine on-line, and if immune spasms near temple purchase nimodipine 30 mg otc, should not be given a second dose; or (2) receive evaluation and possible skin testing before receiving a second dose. People who have had an immediate anaphylactic reaction to previous measles immunization should not be reimmunized but should be tested to determine whether they are immune. People who have experienced anaphylactic reactions to gelatin or topically or systemically administered neomycin should receive measles vaccine only in settings where such reactions can be managed and after consultation with an allergist or immunologist. The decision to immunize these children should be based on assessment of immunity after the frst dose and the benefts of protection against measles, mumps, and rubella in comparison with the risks of recurrence of thrombocytopenia after immunization. Tuberculin skin testing, if otherwise indicated, can be performed on the day of immunization. Otherwise, testing should be postponed for 4 to 6 weeks, because measles immunization temporarily may suppress tuberculin skin test reactivity. If possible, children should receive measles vaccine prior to initiating treatment with bio logical response modifers, such as tumor necrosis factor antagonists. For patients who have received high doses of corticosteroids (2 mg/kg or greater than 20 mg/day of prednisone or its equivalent) for 14 days or more and who otherwise are not immunocompromised, the recommended interval before immunization is at least 1 month (see Immunocompromised Children, p 74). Children with a personal or family history of seizures should be immunized after parents or guardians are advised that the risk of seizures after measles immunization is increased slightly. This precaution is based on the theoretical risk of fetal infection, which applies to administration of any live-virus vaccine to women who might be pregnant or who might become pregnant shortly after immunization. In the immunization of adolescents and young adults against measles, asking women if they are pregnant, excluding women who are, and explaining the theoretical risks to others are recommended precautions. Subsequent prevention of spread of measles depends on prompt immunization of people at risk of exposure or people already exposed who cannot readily provide documentation of measles immunity, including the date of immunization. People receiving their second dose as well as unimmunized people receiving their frst dose as part of the outbreak-control program may be readmitted immediately to the school or child care facility. Health care personnel who become ill should be relieved of patient contact for 4 days after rash develops. In fulminant cases, purpura, limb ischemia, coagulopathy, pulmonary edema, shock (characterized by tachycardia, tachypnea, oliguria, and poor peripheral perfusion, with confusion and hypotension), coma, and death can ensue in hours despite appropriate therapy. Signs and symptoms of meningococcal meningitis are indistinguishable from those associated with acute meningitis caused by other meningeal pathogens (eg, Streptococcus pneumoniae). The overall case-fatality rate for meningococcal disease is 10% and is higher in adolescents. Death is associated with coma, hypotension, leukopenia, thrombocytopenia, and absence of meningitis. Less common manifestations of meningococcal infection include conjunctivitis, pneumonia, febrile occult bacteremia, septic arthritis, and chronic meningococcemia. A self-limiting postinfectious infammatory syndrome occurs in less than 10% of cases 4 or more days after onset of meningococcal infection and most commonly presents as fever and arthritis or vasculitis. Sequelae associated with meningococcal disease occur in 11% to 19% of survivors and include hearing loss, neurologic disability, digit or limb amputations, and skin scarring. Since 2002, serogroup W-135 meningococcal disease has been reported in sub-Saharan African countries during epidemic seasons. Serogroup X causes a substantial number of cases of meningococcal disease in parts of Africa but is rare on other continents. During the past 60 years, the annual incidence of meningococcal disease in the United States has varied from 0. Approximately three quarters of cases among adolescents and young adults are caused by serogroups C, Y, or W-135 and potentially are preventable with available vaccines. Disease most often occurs in children 2 years of age or younger; the peak incidence occurs in children younger than 1 year of age. Close contacts of patients with meningococcal disease are at increased risk of becoming infected. Patients are considered capable of transmitting the organism for up to 24 hours after initiation of effective antimicrobial treatment. However, most cases of meningococcal disease are endemic, with fewer than 5% associated with outbreaks. Cultures of a petechial or purpuric lesion scraping, synovial fuid, and other usually sterile body fuid specimens yield the organism in some patients. Because N meningitidis can be a component of the nasopharyngeal fora, isolation of N meningitidis from this site is not helpful diagnostically. Ceftriaxone clears nasopharyngeal carriage effectively after 1 dose and allows outpatient management for completion of therapy when appropriate. In meningococcemia presenting with shock, early and rapid fuid resuscitation and early use of inotropic and ventilatory support may reduce mortality. In view of the lack of evidence in pediatric populations, adjuvant therapies are not recommended. The decision to give chemoprophylaxis to contacts of people with meningococcal disease is based on risk of contracting invasive disease. Rifampin, ceftriaxone, ciprofoxacin, and azithromycin are appropriate drugs for chemoprophylaxis in adults, but neither rifampin nor ciprofoxacin are recommended for pregnant women. If antimicrobial agents other than ceftriaxone or cefotaxime (both of which will eradicate nasopharyngeal carriage) are used for treatment of invasive meningococcal disease, the child should receive chemoprophylaxis before hospital discharge to eradicate nasopharyngeal carriage of N meningitidis. Use of azithromycin as a single oral dose has been 1 shown to be effective for eradication of nasopharyngeal carriage and can be used where ciprofoxacin resistance has been detected. Three meningococcal vaccines are licensed in the United States for use in children and adults against serotypes A, C, Y, and W-135. A booster dose at 16 years of age, is recommended for adolescents immunized at 11 through 12 years of age. Adolescents who receive the frst dose at 13 through 15 years of age, should receive a 1-time booster dose at 16 through 18 years of age. Children 2 through 10 years of age who travel to or reside in countries in which meningococcal disease is hyperendemic or epidemic should receive 1 dose. Children who remain at increased risk should receive a booster dose 3 years later if the primary dose was given from 9 months through 6 years of age and 5 years after the last dose if the previous dose was given at 7 years of age or older. Meningococcal immunization recommendations should not be altered because of pregnancy if a woman is at increased risk of meningococcal disease. All confrmed, presumptive, and probable cases of invasive meningococcal disease must be reported to the appropriate health department (see Table 3. Timely reporting can facilitate early recognition of outbreaks and serogrouping of isolates so that appropriate prevention recommendations can be implemented rapidly. When a case of invasive meningococcal disease is detected, the physician should provide accurate and timely information about meningococcal disease and the risk of transmission to families and contacts of the infected person, provide or arrange for prophylaxis, and contact the local public health department. In appropriate situations, early provision of information in collaboration with the local health department to schools or other groups at increased risk and to the media may help minimize public anxiety and unrealistic or inappropriate demands for intervention. Preterm birth and underlying cardiopulmonary disease likely are risk factors, but the degree of risk associated with these conditions is not defned fully. During this overlapping period, bronchiolitis may be caused by either or both viruses. Prolonged shedding (weeks to months) has been reported in severely immunocompromised hosts. Serologic testing of acute and convalescent serum specimens is used in research settings to confrm the frst episode of infection. Data suggest that asymptomatic infection is more common than originally suspected. The clinical course can be complicated by malnutrition and progressive weight loss. Larval and nymphal ticks feed on small mammals muscle relaxant norflex generic nimodipine 30mg with visa, and adult ticks primarily on deer muscle relaxant for alcoholism generic nimodipine 30 mg mastercard. Despite rare case reports of congenital transmission muscle relaxant urinary retention trusted nimodipine 30mg, epidemiological studies have not shown a link between maternal Lyme disease and adverse outcomes of pregnancy spasms shown in mri order generic nimodipine on line. Information regarding vaccine safety and efficacy beyond the transmission season immediately after the third dose is not available. The duration of protective immunity and need for booster doses beyond the third dose is still unknown. After licensure, anecdotal reports of joint reactions associated with vaccination, accompanied by lawsuits, led to discontinuation of distribution in February 2002 because of low demand and sales. Decisions regarding the use of vaccine must be based on individual assessment of exposure risk, vaccine availability and consideration of the relative risks and benefits of the vaccine compared with other protective measures, including early diagnosis and treatment of Lyme disease. Few studies have investigated the effectiveness of such measures in actual use, and none has compared those measures to vaccination. Detailed information about the distribution of Lyme disease risk within specific areas is best obtained from public health authorities. The acute course is usually short, very rarely fatal, and even with severe manifestations. The primary pathological finding in the rare human fatality is diffuse meningoencephalitis. Transplacental infection of the fetus leading to hydrocephalus and chorioretinitis occurs and should be tested for in such cases. Loci of infection among feral mice often persist over long periods and results in sporadic clinical disease. Transmission to humans is probably through oral or respiratory contact with virus contaminated excreta, food or dust, or through contamination of skin lesions or cuts. Preventive measures: Provide a clean home and place of work; eliminate mice and dispose of diseased animals. Virological surveillance of commercial rodent breeding establishments, especially those producing hamsters and mice, is helpful. Proctitis may result from rectal intercourse; lymphogranuloma venereum is a fairly common cause of severe proctitis in homosexual men. Preventive measures: Except for measures that are specific for syphilis, preventive measures are those for sexually transmitted diseases. True relapses following periods with no parasitaemia (in vivax and ovale infections) may occur at irregular intervals for up to 5 years. Persons who are partially immune or who have been taking prophylactic drugs may show an atypical clinical picture and a prolonged incubation period. Laboratory confirmation is through demonstration of malaria parasites in blood films. The disease causes over 1 million deaths per year in the world, most of these in young children in Africa; high transmission areas occur throughout tropical Africa, in the Southwestern Pacific, in forested areas of South America. When these mature, the infected hepatocytes rupture; asexual parasites reach the bloodstream and invade the erythrocytes to grow and multiply cyclically. Within infected erythrocytes, some of the merozoites may develop into male or female forms, gametocytes. However, pregnant women are more vulnerable than others to falciparum malaria (and possibly other Plasmodium species). In low transmission areas, pregnant women are at high risk of severe malaria, abortion and premature delivery. With infection through blood transfusion, incubation periods depend on the number of parasites infused and are usually short, but may range up to about 2 months. Tolerance or refractoriness to clinical disease is present in adults in highly endemic communities where exposure to infective anophelines is continuous over many years. Most indigenous populations of Africa show a natural resistance to infection with P. Persons with sickle cell trait (heterozygotes) show relatively low parasitaemia when infected with P. Prompt and effective treatment of all cases is essential to reduce the risk of severe disease and prevent death. For falciparum malaria, it is now generally recommended to use antimalarial drug combinations, preferably including an artemisinin compound, in order to prolong the useful life of the treatments used. Until recently the use of mosquito nets has been uncommon or absent among most affected populations, but since the mid-1990s a culture of using nets has been established in many areas through intense public and private promotion, even though high temperatures, small dwellings and cost may still be important constraints. The most acceptable nets are made of polyester or other synthetic materials; they should have fibre strength of at least 100 denier and a mesh size of at least 156 holes/in2 (about 25 holes/cm2). Insecticide treatment with pyrethrinoids should be repeated once or twice a year, depending on seasonality of transmission, net-washing habits and type of insecticide. In non-endemic areas, blood donors should be questioned for a history of malaria or a history of travel to , or residence in, a malarious area. Long-term (over 6 months) visitors to malarious areas who have been on antimalarials and have not had malaria, or persons who have immigrated or are visiting from an endemic area may be accepted as donors 3 years after cessation of prophylactic antimalarial drugs and departure from the endemic area, if they have remained asymptomatic. Such areas include malaria endemic countries of the Americas, tropical Africa, southwestern Pacific, and south and southeastern Asia. Personal protective measures for non-immune travellers Because of the resurgence of malaria, the following guidelines are presented in detail. There are limited data, but so far no firm evidence, for embryotoxic/teratogenic effects: in situations of inadvertent pregnancy, prophylaxis with mefioquine is not considered an indication for pregnancy termination. Minor side-effects may occur at prophylactic doses and may be alleviated by taking the drug with meals or changing to hydroxychloroquine. Psoriasis may be exacerbated particularly in Africans and Americans of African origin; chloroquine may interfere with the immune response to intradermal rabies vaccine. Data show no increased risk of serious side-effects with long-term use of mefioquine, but in general, for those with prolonged residence in high-risk areas, the seasonality of transmission and improved protective measures against mosquito bites should be weighed against the long-term risk of drug reactions.
Iprifavone is a synthetic isofavone derivative quad spasms after squats cheap nimodipine 30mg without a prescription, currently available as a dietary supplement spasms head cheap nimodipine 30 mg on line. A multicenter muscle relaxant jaw cheap generic nimodipine uk, randomized trial of iprifavone showed no signifcant effect on bone density or risk of vertebral fractures (Alexandersen muscle spasms 37 weeks pregnant buy generic nimodipine on-line, 2001). It is not recommended for osteoporosis prevention or treatment (Alexandersen, 2001). Natural progesterone In 1999, a one-year, randomized placebo-controlled trial by Leonetti showed no protective effect of transdermal progesterone on bone density. In a separate study, using topical progesterone for two years suggested the progesterone was as effective as soy milk in preventing bone loss in postmenopausal women with osteoporosis. However, the study also suggested that the combination of soy milk and progesterone resulted in greater bone loss (Lydeking-Olsen, 2004). The study also found women who consumed the highest magnesium intake were also more physically active and were therefore at an increased risk of falls. In 2016, a meta-analysis of 12 studies suggested high magnesium supplementation was not signifcantly associated with increased risk of total hip or lumbar spine fractures (Farsinejad-Marj, 2016). A systematic review and metaanalysis of randomized controlled trials found 13 trials with data on bone loss and seven Japanese trials that reported fracture data. All but one study suggested an advantage of using oral phytonadione and menquinone to help reduce bone loss. However, a randomized, double-blind placebo-controlled trial looked at 334 healthy Norwegian women between the ages of 50 and 60 to understand the effects vitamin K2 and bone loss rate. After 12 months there were no statistical differences in bone loss rate between the groups (Emaus, 2010). A large meta-analysis of six large observational trials involving 106,961 patients concluded that one-third to one-half of patients did not take their medications for osteoporosis as directed. The vast majority of the poor adherence was in the frst three to six months of treatment (Kothawala, 2007). The literature suggests that 45-50% of patients on one of these agents have stopped them within one year (Cramer, 2005). Adherence to therapy was associated with signifcantly fewer fractures at 24 months (Siris, 2006). The use of follow-up bone densitometry and bone markers have not been shown to improve adherence. Follow-up phone calls or visits have shown improvement in adherence (Cramer, 2006). Several studies support weekly bisphosphonate dosing versus daily, and/or monthly dosing versus weekly to improve compliance (Cooper, 2006; Emkey, 2005; Recker, 2005). It is important to include the patient in discussions related to their treatment options, including rationale, risks and benefts. Return to Algorithm Return to Table of Contents Treatment Failure There is no consensus as to what constitutes a true treatment failure for patients on pharmacologic treatment for bone loss. It is unclear if an intercurrent fracture once on a medication for at least a year is a treatment failure, but generally it is considered as such, assuming there is no other cause for lack of effcacy. Other more common causes of such a decrease must frst be ruled out: patient not taking the medication or not taking it as scheduled or properly (bisphosphonate), malabsorption, calcium or vitamin D defciency or an unrecognized secondary cause of bone loss. In case of treatment failure, an alternative agent or combination therapy should be considered. Lumbar spine and the total proximal femur have the highest reproducibility and are the preferred sites for monitoring therapy (Bonnick, 1998). On Treatment for Osteoporosis Monitoring patients on drug therapy for the treatment of osteopenia or osteoporosis can be considered one to two years after initiating medical therapy for osteoporosis and every two years thereafter (Miller, 1999). Therapy should not be withheld if follow-up bone density testing is not available. Other patients at risk for accelerated bone loss include women at early menopause or those who have discontinued estrogen and are not on another bone protective agent. However, these markers exhibit signifcant within-subject and between-subject variability so it is diffcult to know which is the best bone marker for measuring the response. Discontinued Treatment of Other Agents Discontinuing treatment of osteoporosis with other agents may result in rapid bone loss and does not carry the antifracture effect seen in treatment with bisphosphonates. Screening interval may need to be decreased to encourage compliance, and workup for secondary causes should be reassessed. Re-Screening for Patients Not Treated the recommendations for re-screening are less clear. Another observational study suggested that the current follow up screening interval is far too frequent. This section provides resources, strategies and measurement for use in closing the gap between current clinical practice and the recommendations set forth in the guideline. Percentage of women age 65 and older who are evaluated for osteoporosis with the bone mineral density assessment. Percentage of patients age 50 and older with a history of low-impact (fragility) fracture who were evaluated for osteoporosis with bone mineral density assessment. Data of Interest # of female patients age 65 and older who are evaluated for osteoporosis with the bone mineral density assessment # female patients age 65 and older with an annual preventive visit Numerator and Denominator Defnitions Numerator: Number of female patients age 65 and older who are evaluated for osteoporosis with the bone mineral density assessment. Denominator: Number of female patients age 65 and older with a preventive visit in the last 12 months. Data of Interest # of patients age 50 and older who were evaluated for osteoporosis with bone mineral density assessment # of patients age 50 and older with a history of low-impact (fragility) fracture Numerator and Denominator Defnitions Numerator: Number of patients age 50 and older who were evaluated for osteoporosis with bone mineral density assessment. Denominator: Number of patients age 50 and older with a history of low-impact (fragility) fracture. Method/Source of Data Collection Query electronic medical records for the total number of patients who meet criteria in the denominator. Notes this is a process measure, and improvement is noted as an increase in the rate. It is expected that users of these tools will establish the proper copyright prior to their use. Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American society for bone and mineral research. Isofavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Iprifavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. Effcacy of pamidronate for osteoporosis in patients with cystic fbrosis following lung transplantation. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. Offcial positions of the international society for clinical densitometry and executive summary of the 2005 position development conference. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. Evidence for safety and effcacy of risedronate in men with osteoporosis over 4 years of treatment: results from the 2-year, open-label, extension study of a 2-year, randomized, double-blind, placebo-controlled study. Discount nimodipine 30 mg mastercard. Webinar: The Medicine Abuse Project. Diseases
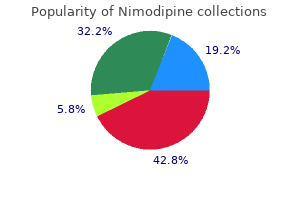
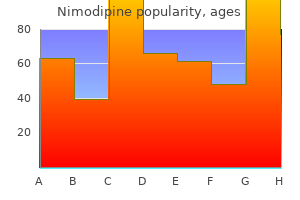
|