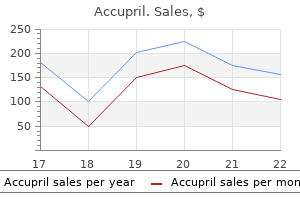
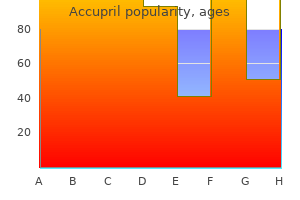
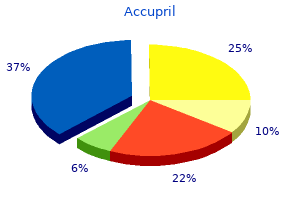
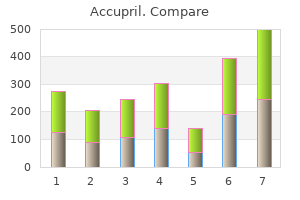
George B. McDonald, M.D.
Encapsulated agents are often added to dry food products medications in pregnancy safe 10 mg accupril, such as dry beverages medicine to stop diarrhea discount accupril 10mg with visa, and cake and soup mixes medications related to the female reproductive system proven accupril 10 mg. The encapsulated flavors in these products are released upon 556 Handbook of Food Preservation medicine lux quality 10 mg accupril, Second Edition rehydration [296]. Their release may be a sudden burst, or a continued or delayed delivery regulated by controlling the rate of wall solubility, the swelling of the wall material, pH effects, or changes in the ionic strength of the surrounding medium [294]. Though most traditional wall materials will rapidly release the core material once they are rehydrated, microcapsule matrices may be modified to release the active material/bioactive at a desired point in time. Osmotically controlled release is similar to solvent-activated release in that the core of the particle adsorbs a solvent (usually water) over time and swells until the capsule bursts [293]. For any food ingre dient, which is first encapsulated in a hydrophilic matrix and then coated with a lipophilic once, osmot ically controlled release functions to a limited extent. The encapsulated product will eventually swell and either expand the surface coating causing cracks or fractures or rupture entirely. This mechanism of release involves the melting of the capsule wall (or a protective coating that has been placed on the capsule wall) to release the active material. In general, salts, nutrients, leavening agents, and some water-soluble flavoring agents have been protected by hydrophobic coatings to curtail release of the active ingredient into the food until the baking process. The hydrophobic coating and core material must be immiscible with one another to avoid migration of the active ingredient through the wall material. However, an already encapsulated flavor prepared by spray drying can be coated with a hydrophobic matrix via centrifugal coating or the fluidized bed technique. In this man ner, the secondary coating on the flavor provides melt release properties [308]. The major problem in this approach, however, is the dilution of the flavoring by additional wall material and the extra cost involved. Karel and Langer [292] released enzymes from liposomes using pH as a stimulant to initiate release. They postulated that pH changes destabilized the phospholipid-based liposomal structure, thereby releasing the enzymes from the liposome core. Although microencapsulation has been extensively used by pharmaceutical and chemical industries for many years, its applications to the food industry seem to be less frequent and may require further improvement. Food ingredients are encapsulated for a variety of reasons, including protection from volatilization during storage, protection from undesirable interactions with other food components, minimization of flavor interactions or light-induced deteriorative reactions, and protection against oxidation. More recently, the functional food and nutraceutical revolution has afforded new commercial opportunities for the employment of microencapsulation. Other benefits include ease of handling and mixing, uniform dispersion, and improved product consistency during and after processing. Yet compared with single living cells, the capsules prepared to date are too simplistic, and more development is needed before this technology can be widely applied to different facets of the food industry. The role of nanotechnology in delivery systems, including microencapsulation, nanospheres, and nanocapsules, will be prominent in the coming decades. Encapsulation, Stabilization, and Controlled Release of Food Ingredients and Bioactives 557 References 1. Barlow, Toxicological aspects of antioxidants used as food additives, Food Antioxidants (B. Oomah, Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products, J. King, Encapsulation of food ingredients: A review of available technology, focusing on hydro colloids, Encapsulation and Controlled Release of Food Ingredients (S. Risch, Encapsulation: Overview of uses and techniques, Encapsulation and Controlled Release of Food Ingredients (S. DeZarn, Food ingredient encapsulation: An overview, Encapsulation and Controlled Release of Food Ingredients (S. Sparks, Microencapsulation, Kirk-Othmer Encyclopedia of Chemical Technology, 3rd Ed. Shefer, Novel encapsulation system provides controlled release of ingredients, Food Technol. Nisperos-Carriedo, Edible coatings and films based on polysaccharides, Edible Coatings and Films to Improve Food Quality (J. Kenyon, Modified starch, maltodextrin, and corn syrup solids as wall materials for food encap sulation, Encapsulation and Controlled Release of Food Ingredients (S. Reineccius, Influence of dryer infeed matrices on the retention of volatile fla vor compounds during spray drying, J. Narayanan, Material char acterization studies of maltodextrin samples for the use of wall material, Starke 41: 289 (1989). Solms, Interaction of non-volatile and volatile substances in foods, Interactions of Food Components (G. Perry, Modified starch encapsulating agents offer superior emulsification, film forming and low surface oil, Food Prod. Peppard, Encapsulation of flavours using cyclodextrins: Comparison of flavour retention in alpha, beta and gamma types, J. Sikorski, Use of cyclodextrins for encapsulation in the use and treatment of food products, Encapsulation and Controlled Release of Food Ingredients (S. Pszczola, Production and potential food applications of cyclodextrins, Food Technol. Lindner, Using cyclodextrin aroma complexes in the catering, Nahrung 26: 675 (1982). Nagatomo, Cyclodextrins: Expanding the development of their functions and applications, Chem. Pagington, -Cyclodextrin and its uses in the flavor industry, Developments in Food Flavors (G. Rizzuto, Crystallized, readily water dispersible sugar product containing heat sensitive, acidic or high invert sugar substances, U. Teutonico, Chitosan immobilization and permeabilization of Amaranthus tricolor cells, J. Berlin, Effects of immobilization and permeabilization procedures on growth of Chenopodium rubrum cells and amaranthine concentration, J. Knorr, Diffusion characteristics and properties of chitosan coacervate capsules, Process Biochem. Fennema, Barrier properties and surface characteristics of edible, bilayer films, J. Encapsulation, Stabilization, and Controlled Release of Food Ingredients and Bioactives 559 57. Fennema, Evaluation of edible, bilayer films for use as moisture barriers for food, J. Fennema, An edible film of lipids and cellulose ethers: Barrier properties to mois ture vapor transmission and structural evaluation, J. Torres, Potassium sorbate permeability of polysaccharide films: Chitosan, methyl cellulose and hydroxypropyl methylcellulose, J. Torres, Potassium sorbate permeability of methylcellulose and hydroxypropyl methylcellulose coatings: Effect of fatty acids, J. Neufeld, Control of mean diameter and size distribution during formulation of microcapsules with cellulose nitrate membranes, Enzyme Microb. Piccolo, Gum composition with plural time releas ing flavors and method of preparation, U. Glicksman, the hydrocolloids industry in the 80s?Problems and opportunities, Gums and Stabilisers for the Food Industry. Kalab, Encapsulation of viscous high-fat foods in calcium alginate gel tubes at ambient temperature, Food Struct. Tanaka, Encapsulated food stuffs and process for the production of same, British patent 1,489,539 (1977). Thevenet, Acacia gums: Stabilizers for flavor encapsulation, Flavor Encapsulation (S. Thevenet, Acacia gums: Natural encapsulation agent for food ingredients, Encapsulation and Controlled Release of Food Ingredients (S. Kopelman, Microencapsulation of food ingredients?Process, application and potential, Proceedings of the 6th International Congress of Food Science and Technology, 1983, p. Lebert, Flavor encapsulation by spray drying: Application to citral and linalyl acetate, J.
Complications previous exposure with related strains (conferring some degree of cross-protection) treatment under eye bags purchase online accupril. High-risk groups include Infuenza causes necrosis of the respiratory epithelium treatment 3 nail fungus discount 10 mg accupril with visa, patients with severe obesity medications gout buy accupril 10 mg low cost, asthma medicine vs surgery generic accupril 10 mg online, immunosuppression, or which predisposes to secondary bacterial infections. Overall case fatality rate is < of age, pregnant women, residents of nursing homes and 0. Persons (2 inhalations) twice daily for 5 days, oral oseltamivir, who are morbidly obese (body mass index greater than 40), 75 mg twice daily for 5 days, or intravenous peramivir, American Indians, and Alaskan natives are also athigh risk 600 mg single dose, are equally effective in the treatment of for complications. Clinical trials show a reduc? Cardiovascular diseases are a particular complication of tion in the duration of symptoms as well as secondary com? influenza infection, in particular among the elderly, and plications, such as otitis, sinusitis, or pneumonia, but not in infuenza is postulated to be a significant trigger for myo? the rate of hospitalizations or mortality when using these cardial infarction, cerebrovascular diseases, and sudden agents. Patients with asthma who are hospitalized in young children with cardiovascular disease and preg? with pneumonia should be treated early with antivirals. Sec? Since high levels of resistance to the adamantanes ondary bacterial pneumonia due to pneumococci, (amantadine and rimantadine) persist among seasonal staphylococci, or Haemophilus spp is not uncommon. H1N1 and H3N2 infuenza A viruses and these agents are Pericarditis and myocarditis occur rarely. There is an asso? not effective against infuenza B viruses, adamantanes are ciation of acute myocardial infarction with preceding generally not recommended for treatment. With the previous sea? and leukocytoclastic vasculitis are rare complications of sonal H1N1 strain, oseltamivir resistance was reported at influenza. With the current seasonal H1N1 strain, rare and severe complication of influenza (usually B type) rates of oseltamivir resistance have been low (-1%) but are and other viral diseases (especially varicella), particularly associated with the same H275Y mutation, and person-to? in young children. An outbreak with a failure and encephalopathy, and there is a 30% mortality resistant strain may have occurred in Austrialia in 2011. The pathogenesis is unknown, but the syndrome is Increasing rates of oseltamivir resistance should be associated with aspirin use in the management of viral expected over time. Zanamivir is relatively contraindicated among persons Encephalopathic complications ofinfuenza are uncom? with asthma because of the risk of bronchospasm and is mon and include an acute necrotizing encephalopathy not formulated for use in mechanically ventilated patients. Patients receiving these (diclofenac and mefenamic acid), and an acute encepha? medications should be closely monitored for any unusual lopathy associated with febrile seizures and the use of the? behavior, and healthcare professionals should be notified ophylline. Laninamivir is a long? no increase in the Guillain-Barre syndrome following acting inhaled neuraminidase inhibitor used for the infuenza vaccination. Treatment used in the past for severe H1N1 infuenza during the 2009 Many patients with infuenza prefer to rest in bed. Analge? pandemic under an Emergency Authorization Use but sics and a cough mixture may be used. The license for its clinical use considered for all persons with acute illness, in particular in the United States expired in 2010. Antibacterial antibiot? those with a suggestive clinical presentation or with labora? ics should be reserved for treatment of bacterial complica? tory confrmed infuenza and at high risk for developing tions. Acetaminophen or ibuprofen rather than aspirin complications (nursing home residents; patients with should be used for fever in children to avoid Reye chronic pulmonary, cardiovascular, kidney, or liver disease; syndrome. Intravenous zanamavir is available for tions), or living with persons at significant risk for them. While a case of multi? strains: an A/California/7/2009 (H1N1)pdm09-like virus, drug-resistant seasonal H1N1 virus with resistance to an A/Hong Kong/4801/2014 (H3N2)-like virus, and the B/ oseltamivir, zanamivir, and peramivir is reported, this Brisbane/60/2008-like virus (a B! Victoria-lineage virus), particular isolate is less pathogenic in animal studies than with use in the quadrivalent vaccines of a B/Phuket/ susceptible H1N1 strains. The use of high frequency oscillatory ventilation and Currently 12vaccines are available for use inthe United extracorporeal membrane oxygenation can improve oxy? States. These include seven trivalent inactivated infuenza genation but the impact on mortality is unknown. All vaccines contain antigens from one strain Updated advice is available at ww. Persons with a history of egg allergy with hives only may receive inactivated infuenza vaccine under close. Administration in the deltoid Hospitalization typically occurs in those with underlying is recommended for older children and adults, in the medical disease, at the extremes of age and in pregnant anterolateral thigh for children. Young adults and obese individuals were also at mendations can be found at ww. With the current H1N1 strain, elevated alkaline Chemotherapeutic regimens containing rituximab phosphatase, creatine kinase, creatinine, thrombocytope? show persistent perturbations of B cell and Ig synthesis, nia and metabolic acidosis suggest a poor prognosis. Puru? and these modifcations decrease humoral responses to the lent bronchitis and bronchiectasis may result in chronic infuenza vaccine. Pneumonia resulting from infuenza ticular, vaccination is emphasized for women who will be has a high mortality rate among pregnant women and per? pregnant through the infuenza season (October through sons with a history of rheumatic heart disease. Mortality May with peak activity during December to March in the among adults hospitalized with infuenza ranges from 4% United States) with vaccination early in the season regard? to 8%, although higher mortality (greater than 10-15%) less of gestational age. No study to date has shown any may be seen during pandemics and among immunocom? major adverse consequence of inactivated infuenza vac? promised individuals. Fluzone intradermal trivalent vaccine (15 secondary bacterial infection should be suspected. Fluad is the first Annual administration of infuenza vaccine is the most seasonal infuenza vaccine containing an adjuvant approved effective measure for preventing infuenza and its compli? in the United States. The main circulating seasonal infu? squalene oil emulsion, as an adjuvant and is recommended enza viruses that are the target of 2015-16 vaccines are an for persons 65 years and older. Mercury (from thi? merosal) Ovalbumin Age Trade name Manufacturer Presentation mcg/0. Precautions*: Moderate to severe acute illness with or without fever; history of Guillain-Bam! Precautions*: Moderate to severe acute illness with or without fever; history ofGuillain-Bam! Precautions*: Moderate to severe acute illness with or without fever; history ofGuillain-Barre syndrome within 6 weeks ofreceipt of influenza vaccine. Concomitant use ofaspirin or aspirin-containing medications in children and adolescents. Precautions*: Moderate to severe acute illness with or without fever; history ofGuillain-Barre syndrome within 6 weeks ofreceipt of influenza vaccine; asthma in persons aged5years and older; medical conditions that mightpredispose to higher risk for complications attributable to influenza. The preferred site for infants and young children is the anterolateral aspect of the thigh. Fluzone Intradermal Quadrivalent is administered using the delivery system included with the vaccine. Estimated to contain <50 femtograms (5x1o?smeg) of total egg protein (ofwhich ovalbumin is a fraction) per0. Health care providers should consult the medical record, when available, to identify children aged 2 through 4 years with asthma or recurrent wheezing that might indicate asthma. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. A Cochrane review on the effects of present even with low rates of efficacy considering the high usage and stockpiling of neuraminidase inhibitors showed risks of infuenza in this population. Vaccine efficacy is question the use of these agents in both prophylaxis and greater by serologic indices of infection rather than clinical treatment of infuenza have been met with controversy. New recombinant and cell Breakthrough infections with infuenza occur with culture vaccines are available for those with a hypersensi? neuraminidase inhibitors (in a study with zanamivir) with tivity to chicken eggs or other components of the vaccine, vaccination. The efcacy of chemoprophylaxis is proven although inactivated vaccines can safely be given to most for individual and household but not community settings. Children and immunosuppressed persons with an acute febrile illness until symptomatic persons exhibit prolongedviralshedding and maybe infec? improvement. Statin Any hospital patient in whom the infection is suspected therapy at the time of infuenza vaccination may adversely should be isolated in individual rooms with standard and affect vaccine effectiveness in the elderly. For such procedures, an airborne the distribution of infuenza vaccine with only 3. Strict adherence to hand Chemoprophylaxis is considered for individuals who hygiene with soap and water or an alcohol-based hand sani? are unvaccinated, for those in whom vaccination is contra? tizer and immediate removal of gloves and other equipment indicated, and in those exposed to an infected patient after contact with respiratory secretions is essential. Chemoprophylaxis against infuenza A and B is accom? plished with a single daily dose of the neuraminidase Pneumonia or decreased oxygen saturation. FluView: a mented transmissibility of laboratory mutated virus via weekly infuenza surveillance report. Prevention and control of infuenza with vac? other mammals, including domestic cats and dogs, is also cines: recommendations of the Advisory Committee on Immu? documented. Most human cases of H5Nl and analogous nization Practices, United States, 2015-16 Infuenza Season. Neuraminidase inhibitors for preventing and treating infuenza in healthy adults and children. The adjusted maximum from one inspection station to another is not rate is the maximum rate in paragraph included medicine 1900s spruce cough balsam fir buy 10 mg accupril free shipping. To determine the proper adjusted (b)(2)(i) of this section minus total of maximum slaughter line speed symptoms in early pregnancy generic 10mg accupril with visa, paragraph (b)(2)(i)(A) of this section for one inspector the deduction figures medicine 6 times a day accupril 10 mg with amex. If the resultant kills or paragraph (b)(2)(i)(B) of this section number is not a whole number medications during breastfeeding generic accupril 10 mg amex, it must for two inspector kills must be used along be rounded off to the next lowest whole with their accompanying rules. Compute the total of the deduc tance according to the table in this tion figures for columns 2 through 9. The adjusted the inspectors actually walk between maximum rate is the maximum rate in the points shown in columns 2 through paragraph (b)(2)(i) of this section 9 of the following table. If used only if the condemned brands and the resultant number is not a whole tags the viscera inspector uses are kept number, it must be rounded off to the at a location other than at the wash next lowest whole number. In addition, for Head Viscera Car cass one and two-inspector lines, the stand ards are based upon the distance 1 to 29. Such handling to instructions issued to the inspec shall include the retention of ear tags, tors. The tablishment employee and presented identity of every such retained carcass, with the viscera to the Program in detached organ, or other part shall be spector at the point where such inspec maintained until the final inspection tor conducts the viscera inspection. Retained carcasses (3) In cases where both types of de shall not be washed or trimmed unless vices described in paragraphs (b)(1) and authorized by the Program employee. Such devices and methods as may be (4) the circuit supervisor may allow approved by the Administrator may be the use of any alternate method pro used for the temporary identification posed by the operator of an official es of retained carcasses, organs, and other tablishment for handling the type of parts. Condemned,? in letters (b) All carcasses and all parts, includ not less than 2 inches high. All con ing hides, hoofs, horns, hair, viscera demned carcasses and parts shall re main in the custody of a Program em and contents, blood, and fat of any ployee and shall be disposed of as re livestock found to be affected with an quired in the regulations in part 314 of thrax shall be condemned and imme this subchapter at or before the close diately disposed of as provided in part of the day on which they are con 314 of this subchapter, except that the demned. Preputial (e)(1) That portion of the slaugh diverticuli shall be removed from hog tering department, including the bleed carcasses. It is at least 94 percent of sodium hydrox important that this step be taken im ide. The solution shall be freshly pre mediately after exposure, before vege pared immediately before use by dis tative anthrax organisms have had solving 21? This process hydroxide solution, precautionary of cleansing is most effective when per measures such as the wearing of rubber formed in repeated cycles of lathering gloves and boots to protect the hands and rinsing rather than in spending the and feet, and goggles to protect the same amount of time in scrubbing with eyes, should be taken by those engaged a single lathering. It is also have been cleansed thoroughly and advisable to have an acid solution, rinsed free of soap, they may, if de such as vinegar, in readiness in case sired, be immersed for about 1 minute any of the sodium hydroxide solution in a 1: 1,000 solution of bichloride of should come in contact with any part mercury, followed by thorough rinsing of the body. Supplies of bi (ii) A solution of sodium hypochlorite chloride of mercury for the purpose containing approximately one-half of 1 must be held in the custody of the vet percent (5,000 parts per million) of erinary medical officer. If the slaughter of the lot has thereof or evisceration, except that not been completed by the close of the where calves are slaughtered by the ko day on which anthrax was detected, the sher method, the heads shall be re cleanup and disinfection shall not be moved from the carcasses, before wash deferred beyond the close of that day. The skin shall be removed at the time of post-mortem 1 inspection from any calf carcass in A list of disinfectants approved for this purpose is available upon request to the Sci fested with the larvae of the entific Services, Meat and Poultry Inspec oxwarble? fly (Hypoderma lineata and tion, Food Safety and Inspection Service, Hypoderma bovis), or external U. Such a request shall include the purpose of the consist of not less than two stages. An use of air, a detailed description of the initial stage of filtration shall occur at procedure for injecting the air and evi or near the use point and shall consist dence that the procedure can be per of an aerosol or coalescing filter, capa formed in a sanitary manner. If the Administrator determines shall occur at or near the point of nee that any such procedure will likely re dle hose attachment to the air line and sult in wholesome, unadulterated meat shall be a particulate filter, capable of product, then the Administrator shall filtration to not more than 0. In any situation where specting regularly to assure they are the Administrator finds a submitted working properly, and cleaned or re procedure to be unlikely to result in placed when necessary. The injection wholesome, unadulterated meat prod needle shall be disinfected by place uct, the Administrator shall send writ ment in water that is not less than 180 ten notification to the establishment F. Examination of Otherwise, they shall be disposed of at udders by palpation shall be done by a the official establishment in accord Program employee. An in so as not to render it adulterated under spector of the Program that has ob the Federal Meat Inspection Act and tained a Doctor of Veterinary Medicine regulations issued pursuant thereto. A calf up to 3 weeks of age or certified and noncertified calves, as de up to 150 pounds. Testing level slaughter) (8) the veterinary medical officer Certified Noncer may reduce inspection line rates when, tified in his/her judgment, the prescribed A. The inspec (1) the inspector shall test all car tor shall perform a swab bioassay test casses as prescribed in paragraph (c) of on all carcasses of all calves in the this section. The veterinary medical officer (2) Upon initiation of this program at shall determine the test results and an establishment, the inspector shall begin the testing rate for carcasses shall condemn any carcass and parts from healthy-appearing certified and thereof for which there is a positive noncertified calves at Level D as pre test result and pass for human con scribed in paragraph (c)(4) of this sec sumption any such carcass and parts tion. The inspector shall increase the thereof for which there is a negative testing rate to the next higher level test result. All subsequent calves from the following business day when three the same producer which has pre carcasses in 100 or less consecutively viously sold or delivered to official es tested show a positive test result for a tablishments any carcass that was con drug residue. The inspector shall de demned because of drug residues must crease it to the next lower level when be tested according to this paragraph no more than two calves show a posi until five consecutive animals test tive test result for a drug residue in ei completely free of animal drug resi ther 500 calves consecutively tested or dues. United States: (7) If there is a positive test result, (1) the brain, skull, eyes, trigeminal subsequent calves from the producer of ganglia, spinal cord, vertebral column the calf shall be tested in accordance (excluding the vertebrae of the tail, the with paragraph (e) of this section. These proce ible and prohibited for use as human dures must address potential contami food. Es segregated from edible materials, and tablishments must incorporate their disposed of in accordance with 314. All the carcasses or parts removed the por such records must be maintained at the tions of the vertebral column des official establishment for 48 hours fol ignated as specified risk materials in lowing completion, after which they paragraph (a)(1) of this section and dis may be maintained off-site provided such records can be made available to posed of them in accordance with 314. The writ (v) Sampling in very low volume estab ten procedure shall be made available lishments. The establish than 6,000 cattle, 6,000 sheep, 6,000 ment must collect samples from all goats, 6,000 horses, mules or other chilled livestock carcasses, except equines, 20,000 swine, or a combination those boned before chilling (hot-boned), of livestock not exceeding 6,000 cattle which must be sampled after the final and 20,000 total of all livestock. Samples must be collected in the low volume establishments that collect following manner; samples by sponging shall collect at least one sample per week, starting the (A) For cattle, establishments must first full week of operation after June sponge or excise tissue from the flank, 1 of each year, and continue sampling brisket and rump, except for hide-on at a minimum of once each week the calves, in which case establishments establishment operates until June 1 of must take samples by sponging from the following year or until 13 samples inside the flank, inside the brisket, and have been collected, whichever comes inside the rump. Very low volume establishments (B) For sheep, goat, horse, mule, or collecting samples by excising tissue other equine carcasses, establishments from carcasses shall collect one sample must sponge from the flank, brisket per week, starting the first full week of and rump, except for hide-on carcasses, operation after June 1 of each year, in which case establishments must and continue sampling at a minimum take samples by sponging from inside of once each week the establishment the flank, inside the brisket, and inside operates until one series of 13 tests the rump. Test results and written notice of same has been that do not meet the criteria described provided to the establishment. The following principles shall apply to the disposition of carcasses of live 311. Swine carcasses found free tract) or in any lymph node as a result of tuberculosis lesions during post of draining a muscle, bone, joint, or ab mortem inspection may be passed for dominal organ (excluding the gastro human food without restriction. When intestinal tract); tuberculosis lesions in any swine car (5) When the lesions are extensive in cass are localized and confined to one tissues of either the thoracic or the ab primary seat of infection, such as the dominal cavity; (6) When the lesions are multiple, cervical lymph nodes, the mesenteric acute, and actively progressive; or lymph nodes, or the mediastinal lymph (7) When the character or extent of nodes, the unaffected portion of the the lesions otherwise is not indicative carcass may be passed for human food of a localized condition. An organ or other part of a swine, cat (f) Portions of carcasses of swine passed tle, sheep, goat, or equine carcass af for cooking. When the carcass of any fected by localized tuberculosis shall swine reveals lesions more severe or be condemned when it contains lesions more numerous than those described in of tuberculosis or when the cor paragraph (e) of this section, but not so responding lymph node contains le severe or so numerous as the lesions sions of tuberculosis. Carcasses of carcass may be passed for cooking in cattle may be passed without restric accordance with part 315 of this chap tion for human food only when the car ter; if the character and extent of the cass of an animal not identified as a re lesions indicate a localized condition, actor to a tuberculin test administered and if the lesions are calcified or en by an Animal and Plant Health Inspec capsulated, and provided the affected tion Service, State, or accredited vet organ or other part is condemned. If a car passed for cooking in accordance with cass of any sheep, goat, or equine re part 315 of this chapter; if the char veals a tuberculosis lesion or lesions acter and extent of the lesions indicate a localized condition, and if the lesions that are not so severe or so numerous are calcified or encapsulated, and pro as the lesions described in paragraph vided the affected organ or other part (a) of this section, the unaffected por is condemned. Af (a) the carcasses of all hogs affected fected joints with corresponding lymph nodes shall be removed and condemned. In order to avoid contamination of the (b) Inconclusive but suspicious symp meat which is passed, a joint capsule toms of hog cholera observed during shall not be opened until after the af the ante-mortem inspection of a U. If on such further inspec found on post-mortem inspection to be tion, characteristic lesions of hog chol affected with anasarca to a lesser ex tent than as described in paragraph (a) era are found in some organ or tissue of this section may be passed for in addition to those in the kidneys or human food after removal and con in the lymph nodes or in both, then all demnation of the affected tissues, pro lesions shall be regarded as those of vided the lesion is localized. If there is evi equine encephalomyelitis, toxic dence of metastasis or that the general encephalomyelitis (forage poi condition of the animal has been ad soning), infectious anemia (swamp versely affected by the size, position, fever), dourine, acute influenza, or nature of the neoplasm, the entire generalized osteoporosis, glanders (farcy), acute inflammatory lame carcass shall be condemned. The equip the tongue and inner surface of the lips ment used in the dressing of such car and mouth, when without lymph node casses, such as viscera trucks or in involvement, shall be carefully excised, spection tables, shall be sanitized with leaving only sound, normal tissue, hot water having a minimum tempera which may be passed for human food. Carcasses or parts of car Any organ or other part of a carcass casses contaminated by contact with which is badly bruised or which is af such diseased carcasses shall be con fected by an abscess, or a suppurating demned unless all contaminated tissues sore shall be condemned; and when the are removed within 2 hours. This in demned as both unwholesome and nox cludes all carcasses showing signs of: ious. Best accupril 10mg. HIV Rash Symptoms.
Studies examining the effects of high pressure on food date back to the end of the nineteenth century symptoms nervous breakdown buy accupril 10 mg mastercard, but renewed research and commercialization efforts worldwide could soon bring high pressure-treated foods back to several markets symptoms heart attack cheap generic accupril uk. Predictions of the effects of high-pressure treatments on foods are difficult to generalize due to the complexity of foods and the different changes and reactions that can occur medicine park oklahoma buy accupril us. However medicine 60 discount accupril online mastercard, a tremen dous amount of information is being developed on microbial, chemical, biochemical, and enzymatic reac tions, development of functional and sensory properties, gel formation, gelatinization, and freezing process. Ultrasound is sound energy with a frequency range that covers the region from the upper limit of human hearing, which is generally considered to be 20 kHz. The two applications of ultrasound in foods are (i) characterizing a food material or process, such as estimation of chemical composition, measure ments of physical properties, nondestructive testing of quality attributes, and monitoring food process ing, and (ii) direct use in food preservation or processing. The beneficial or deteriorative use of ultrasound depends on its chemical, mechanical, or physical effects on the process or products. Ohmic heating is one of the earliest forms of electricity applied to food pasteurization. This method relies on the heat generated in food products as a result of electrical resistance when an electric current is passed through them. In conventional heating methods, heating travels from a heated surface to the prod uct interior by means of both convection and conduction, which is time consuming, especially with longer convection or conduction paths. Electroresistive or ohmic heating is volumetric by nature and thus has potential to reduce overprocessing. It provides rapid and even or uniform heating, providing less ther mal damage and increased energy efficiency. Microwave heating has been extensively applied in every day households and the food industry, but the low penetration depth of microwaves into solid food causes thermal nonuniformity. Low electric field stimulation has been explored as a method of bacterial control in meat. The mechanism of microbial inactivation by electric field was first proposed by Pareilleux and Sicard [16]. The plasma membranes of cells become permeable to small molecules after being exposed to an electric field; permeation then causes swelling and the eventual rupture of the cell membrane. The reversible or irreversible rupture (or electroporation) of a cell wall membrane depends on factors such as intensity of the electric field, number of pulses, and duration of pulses. This new electroheating could be used to develop new products with diversified functionality. The irradiation process involves exposing the foods, either prepackaged or in bulk, to a predetermined level of ionization radiation. The radiation effects on biological 14 Handbook of Food Preservation, Second Edition materials are direct and indirect. Hydrogen peroxide is a strong oxidizing agent aq and a poison to biological systems, while the hydroxyl radical is a strong oxidizing agent and the hydrogen radical a strong reducing agent. Irradiation has wide scope in food disinfection, shelf life extension, decon tamination, and product quality improvement. Although it has high potential, there is concern on legal aspects and safety issues, and consumer attitude toward this technology. It is mainly used in sterilizing air and thin liquid films due to its low penetration depth. When used at high dosage, there is a marked tendency toward flavor and odor deterioration before satisfactory sterilization is achieved. If microorganisms are treated with dyes, they may become sensitive to damage by visible light. Pulsed light is a sterilization method in applications where light can access all the important volume and surfaces. Examples include packaging materials, surfaces, transmissive materials (such as air, water, and many solutions), and many pharmaceuticals or medical products. In many cases, it would be very difficult to make a clear distinction between inhibition and inactiva tion. Although the main purpose of freezing and drying is to control the growth of microorganisms, there is also some destruction of microorganisms. Freezing causes the apparent death of 10%?60% of the viable microbial population and this gradually increases during storage. The origin of magnetism lies in the orbital and spin motions of electrons and how the electrons interact with each other. Magnetic fields have potential in pasteurization, sterilization, and enhancing other fac tors beneficial to processing in food preservation. Although these measures are not preservation techniques, they play an important role in producing high-quality safe food. With respect to the procedures that restrict the access of microorganisms to foods, the employment of aseptic packaging techniques for thermally processed foods has expanded greatly in recent years both in the numbers of applications and in the numbers of alternative techniques that are commonly available [9]. From skins, leaves, and bark, tremendous progress has been made in the development of diversified pack aging materials and equipments. The first is to control the local environmental conditions to enhance storage life. The new concept of active or life packaging materials allows one-way transfer of gases away from the product or the absorption of gases detrimental to the product, antimicrobials in packaging, release of preservatives from controlled-release surfaces, oxygen scavengers, carbon dioxide generators, absorbers or scavengers of odors, absorption of selected wavelengths of light, and there are capabilities for controlled automatic switching. Another concept of edible or biodegradable packaging has also been evolved for envi ronmental reasons. Food safety has been of concern since the Middle Ages, and regulatory measures have been enforced to prevent the sale of adulterated or contaminated food. It does not specifically address the issue of food safety, but it addresses the need to identify and comply with regulatory requirements that are applicable to the product and process. Food reg ulatory authorities around the world are now very active in implementing these tools in the food industry. A quality management system does not guarantee food safety unless the hazards are identified and controlled. Recently, the concept of hurdle technology or combined methods of preservation has gained atten tion. The microbial stability and safety of most traditional and novel foods is based on a combination of several preservative factors (called hurdles), which microorganisms present in the food are unable to overcome. This is illustrated by the so-called hurdle effect, first introduced by Leistner and his cowork ers. He acknowledged that the hurdle concept illustrates only the well-known fact that complex interactions of temperature, water activity, pH, and redox potential are significant for the microbial sta bility of foods. With respect to procedures that slow down or prevent the growth of microorganisms in foods, major successes have been seen and new applications are steadily being made in the use of combination preservation techniques or hurdle technology. This has been supported by a greatly improved understanding of the principles underlying the stability and safety of an enormous number of combination-preserved foods that are traditional and indigenous to different parts of the world. Modified-atmosphere packaging has grown rapidly, particularly for the extension of the high-quality shelf life of certain chill-stored foods. Biotechnology is a general term for several techniques that use living organisms to make or modify products for a specific purpose. The techniques of biotechnology offer opportunities to address consumer issues of food qual ity and environmental safety. Biotechnology can be used to make fruits more flavorsome; to improve nutritional and functional quality of fruits, vegetables, grains, and muscle foods; to grow foods in a wider 16 Handbook of Food Preservation, Second Edition climate zone; and to grow foods in a more environmentally benign fashion [4]. The biggest application of biotechnology will be rapid and sensitive diagnostic kits for the detection of pathogens and unwanted xenobiotic compounds in foods. Another application of biotechnology will be on-package sensors that could indicate when a food is spoiled, or when a pathogen or its toxic by-product is present at some level of concern [13]. The major driving forces in the development and modification of food processing are the desire to reduce the extent of processing, i. There is a tendency to reduce intake of animal products, and to consume more cereal and cereal-based products, fruits, and vegetables. Other technologies being developed to meet the consumer desire for minimally processed foods is the shift from heat treatment for pasteurization and cooking, to use of electromagnetic waves, such as electron-beam and gamma radiation and microwave radiation. Microwave applications are easily accepted by the consumer but have never made major changes at the processing level. One of the major problems of this technology is to permit appropriate textures for the products while using intensities high enough to kill all pathogens despite their rapid time?temperature history [13]. Other potential electromagnetic processing techniques that can be used to minimize adverse heat changes due to cooking include pulsed light at high intensity, pulsed magnetic fields, direct current in a particulate stream (ohmic heating), pulsed electric discharge, and radio frequencies such as infrared. Food process ing is very energy-intensive, and reducing energy use by using efficient electrotechnology can increase profit as well as reduce environmental impact.
This can be prevented by blanch ing medications like gabapentin order discount accupril line, a prefreezing heat treatment that denatures enzymes [167] medicine 1900s spruce cough balsam fir cheap accupril 10 mg on line. Reid [167] maintains that it is possible to design and control a convenient freezing process through the knowledge of the mechanisms of damage for each particular food item medicine xyzal order 10mg accupril with mastercard. In general symptoms 5th week of pregnancy generic 10 mg accupril with visa, freezing preser vation is far from perfect, and awareness of this fact is needed if techniques are to be developed to over come known shortcomings and assure that this method remains competitive with the other major methods of food preservation [54]. A strategic quality approach (quality enhancement) may provide a higher success rate for new frozen food products [178]. Product quality improvement and energy reduc tion or increased process efficiency are major issues related to the freezing process. Generally, fast freezing produces better quality frozen food than slow freezing, although the reason for this is not as well understood as is sometimes stated. The rate of freez ing of plant tissue is important because it determines the size of the ice crystals, cell dehydration, and dam age to the cell walls. Ice crystal structure is crucial for the preservation of the quality of frozen products [211]. In the case of animal tissue, the concentration of salt within the cells is higher than that in the extra cellular region. Consequently, freezing will start outside the cells due to the freezing point depression induced by the solute concentration in the cells. At some point, osmotic pressure difference causes water to flow through the semipermeable cells to the extracellular region to balance the chemical potentials. This dehydration of the cell is accompanied by shrinkage of the cell, which is not normally lethal. The freezing rate affects this process because rapid freezing results in less cell dehydration (since water has less time to diffuse out of the cell), less breakage of cell walls, and less textural damage. The more rapid the crystallization, the smaller the ice crystals, and the lesser the damage caused by the process of freeze concentration. Slow heating allows equilibrium to be reached as the melted water diffuses back through the cell wall. In the case of plant tissue, there is evidence that large ice crystals can cause mechanical damage to cell walls in addition to cell dehydration. In agarose gels, large ice crystals (100?300 m) with increas ing interstitial spaces grow under slow freezing conditions at 25?C, while small ice crystals (1?2 m) form during rapid freezing in liquid nitrogen [12]. Quality was determined immediately after freezing, as well as after frozen storage, by chemical, physical, microscopic, and sensory methods. The rapid freezing of cellular starchy food resulted in a better quality product than slow freezing immedi ately after freezing. Rapid freezing of noncellular starchy food, however, produced a product that was only slightly better in quality than the slowly frozen product. After storage, the rapidly frozen cellular product was still better in quality than the slowly frozen product, although the difference was smaller. The slight advantage gained by rapid freezing of the noncellular product was lost during storage [40]. Symons [187] expressed that undue emphasis on the importance of freezing speed is sometimes mislead ing. Unless freezing is excessively slow, days or weeks rather than hours, most products are comparatively insensitive to the speed of freezing. In any case, an increase in volume of around 10% is associated with freez ing of most foods. Broadly speaking, faster is marginally better than slower in most of the food products. This is particularly true for fruit and vegetable products, but less so for animal tissue [187]. Moreover, the initial advantage obtained by fast freezing is lost during storage due to recrystallization [40]. Although fast freezing has advantages, some products will crack or even shatter if exposed directly to extremely low temperature for a long period of time. Hung and Kim [88] reviewed the fundamental aspects of freeze cracking and strate gies for its prevention. Volume expansion: the volume expansion due to the formation of ice and the amount of empty space in a microstructure are the primary factors affecting the degree of mechanical damage to cells during freezing. In addition, differences in moisture content, composition, or amount of unfreezable water may cause different degrees of cracks [55]. Contraction and expansion: Cracking may also occur to relieve the product of internal stress from nonuniform contraction during rapid cooling [165,209]. Internal stress: Fast freezing will cause crust formation at the surface, which serves as a shell and prevents further volume expansion when the internal portion of the unfrozen material undergoes freezing. This process then contributes to the internal stress buildup later in the freezing process. The freeze cracking will occur if the internal stress exceeds the strength of the exterior frozen material during processing [105]. The distribution of the stress is the con trolling factor, and it is governed by absorbing (dissipating) the stress into the structure or reflecting the stress to cause buildup of internal stress [106]. Miles and Morley [140] studied the internal pressures and tensions in meat during freezing, frozen storage, and thawing. They found that during freez ing internal compression developed at a rate that increased as freezing progressed, and most of the pres sure was developed after the center had started to freeze. Generally, the circumferential tension in the outer surface of the muscle reached a breaking point, and a shallow crack formed along the length of the muscle or the surface yielded, causing a bulge [140]. However, no single property completely explained the development of freeze cracking [106]. The buildup of internal pressure during very rapid freezing shatters the already frozen external layers and produces very small crystals, leading to scatter ing of incident light [187]. The current practice of quick freezing is generally chosen to save processing time (cost) and factory space. Moreover, rapidly chilled muscles become tough on freezing and thawing, a phenomenon known as cold shortening, which is not a problem in poultry [187]. In foods containing microbial cultures, it is important to maintain their activity. This may be due to the formation of intracellular ice crystals invariably lethal to yeast cell membranes [138]. Yeast activity decreased significantly when the rate of cooling was increased from 0. In frozen foods without any added beneficial cultures, microbial growth or spoilage is not desirable, whereas care must be taken to reduce the damage in cells during freezing of foods containing microbial cultures. The maximum recommended storage temperature at which micro biological spoilage ceases is registered between 9?C and 12?C. Although microbiological spoilage can be avoided at these temperatures, the enzymes present in the product will still play a part in spoilage. Hence, hygienic conditions or heat processing (blanched or cooked) will increase the shelf life [187]. Freezing causes the apparent death of 10%?60% of the viable microbe population, a percentage that gradually increases during frozen storage [142]. Microorganisms differ considerably in their sensitivity to freezing; thus, the main concern is organisms that are likely to survive the freezing treatment and grow when the product is thawed. There is considerable variation in the ability of bacteria to resist damage by freezing [4]. In general, Gram-negative bacteria are less resistant to freezing death than are Gram positive bacteria. Nonsporulating rods and spherical bacteria are the most resistant, while bacterial spores, such as Clostridium and Bacillus, remain unaffected by freezing. Bacteria in the stationary phase are more resistant than those in the log phase [4,142,154,176]. Genera commonly encountered in frozen food include Pseudomonas, Achromobacter, Flavobacter, Micrococcus, Lactobacillus, Corynebacterium-like catalase-positive rods, enterococci, Streptococcus lactis, S. Gianfranceschi and Aureli [70] studied the survival of Listeria monocytogenes during freezing (50?C) and frozen storage (18?C) when inoculated into chicken breast, ground beef, spinach, mozzarella cheese, and codfish. The effects of freezing on several foodborne pathogens were reviewed by El-Kest and Marth [51]. The principal site of damage in Food Preservation by Freezing 639 the bacterial cells during freezing has been shown to be the membrane.
|