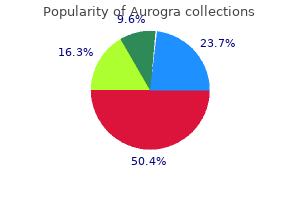
Gary W. Chune, MD
Bayer is seeking indemnification for 2019 erectile dysfunction pills uk aurogra 100 mg for sale, subject to an extension for claims related to certain types damages allegedly suffered in several hundred individual of product liability notified before such date erectile dysfunction treatment kolkata buy generic aurogra. Aventis Animal Nutrition Retained Liabilities Aventis CropScience Retained Liabilities Aventis Animal Nutrition S erectile dysfunction medicine reviews aurogra 100 mg with mastercard. The representations and warranties and the indemnification are indemnification undertakings are subject to an overall cap of subject to a cap of 836 million erectile dysfunction medicine purchase aurogra without a prescription, except for certain legal 223 million, with a lower cap for certain environmental claims. There are various periods of limitation depending upon the nature or subject of the the demerger of the specialty chemicals business from Hoechst indemnification claim. Under the demerger agreement between Hoechst and Celanese, Hoechst expressly excluded Since December 2005, Aventis Agriculture and Hoechst GmbH any representations and warranties regarding the shares and have concluded several settlement agreements to resolve a assets demerged to Celanese. Thereafter, Celanese remains liable for known from certain locally concentrated pollutions in the sites taken over environmental claims specified in 2013. In January 2004, two minority shareholders of Rhodia and their Infraserv Hochst Retained Liabilities respective investment vehicles filed two claims before the Commercial Court of Paris (Tribunal de Commerce de Paris) By the Asset Contribution Agreement dated December 19/20, 1996, against Aventis, to which Sanofi is successor in interest, together as amended in 1997, Hoechst contributed all lands, buildings, and with other defendants including former directors and statutory related assets of the Hoechst site at Frankfurt Hochst to Infraserv auditors of Rhodia from the time of the alleged events. Infraserv Hochst undertook to indemnify claimants seek a judgment holding the defendants collectively Hoechst against environmental liabilities at the Hochst site and with liable for alleged management errors and for alleged publication respect to certain landfills. These shareholders seek a finding of joint reimburse current and future Infraserv Hochst environmental and several liability for damages to be awarded to Rhodia in an expenses up to 143 million. As a former operator of the land and amount of 925 million for alleged harm to it (a derivative action), as a former user of the landfills, Hoechst may ultimately be liable for as well as personal claims of 4. In 2006, the Commercial Court of Paris related to liabilities arising before Closing. Sanofi is working to investigate the validity of decision before the French Supreme Court (Cour de cassation). Provisions for discounts, rebates and sales returns Adjustments between gross sales and net sales, as described in Note B. Personnel costs Total personnel costs include the following items: ( million) 2018 2017(a) 2016(a) Salaries 6,547 6,592 6,424 Social security charges (including defined-contribution pension plans) 1,954 1,977 1,948 Stock options and other share-based payment expense 282 258 250 Defined-benefit pension plans 261 275 273 Other employee benefits 225 219 224 Total 9,269 9,321 9,119 (a) Excluding personnel costs for the Animal Health business: immaterial in 2017 and 0. The total number of registered employees (excluding those of the compared with 106,566 as of December 31, 2017 and 106,859 Animal Health business) was 104,226 as of December 31, 2018, as of December 31, 2016. Other operating income In 2018, this line item includes 225 million of expenses relating to the agreement with Regeneron, versus 11 million in 2017 and Other operating income totaled 484 million in 2018, versus 10 million in 2016. In 2017, Sanofi recognized an impairment mature products in Latin America and some Consumer loss of 87 million against property, plant and equipment Healthcare products in Europe (326 million in 2018, 90 million associated with the dengue vaccine project. Other operating expenses totaled 548 million in 2018, compared with 233 million in 2017 and 482 million in 2016. Restructuring costs and similar items Restructuring costs and similar items amounted to 1,480 million in 2018, 731 million in 2017 and 879 million in 2016, and comprise the following items: ( million) 2018 2017 2016 Employee-related expenses 517 336 650 Expenses related to property, plant and equipment and to inventories 162 221 139 Compensation for early termination of contracts (other than contracts of employment) 352 61 31 Decontamination costs 5 (4) 3 Other restructuring costs 444 117 56 Total 1,480 731 879 Restructuring costs recognized in 2018 included: (b)a provision of 283 million booked as of December 31, 2018 for penalties arising from the restructuring of the (a)termination benefit payments of 517 million in 2018, immuno-oncology research and development agreement including provisions associated with the headcount with Regeneron, and in particular on termination of the adjustments in Europe announced in December 2018. Other gains and losses, and litigation (d)the costs of transferring the infectious diseases early stage In 2018, the line item Other gains and losses, and litigation R&D pipeline and research unit. Those transfer costs consists of the pre-tax gain of 502 million arising on the amounted to 252 million and primarily consist of payments divestment of the European Generics business (completed to Evotec over a five-year period, including an upfront September 30, 2018), net of separation costs (see Note D. Consequently, Sanofi recognized an impairment loss reflecting the difference between the historical acquisition cost of its shares in Alnylam and their market value. In 2018, 2017 and 2016, the impact of the ineffective portion of hedging relationships was not material. Income tax expense Sanofi has elected for tax consolidations in a number of countries, principally France, Germany, the United Kingdom and the United States. The difference between the effective tax rate and the standard corporate income tax rate applicable in France is explained as follows: (as a percentage) 2018 2017 2016(a) Standard tax rate applicable in France 34. In 2016, entities subject to corporate income tax in France were liable to pay an additional tax contribution in respect of amounts distributed by the entity. In determining the amount of the deferred tax liability for 2018, 2017 and 2016, Sanofi took into account changes in the ownership structure of certain subsidiaries. For 2016, it includes the effects of changes in tax rates in various countries, particularly in France, Hungary, Italy, Japan and the United States. For the periods presented, the amount of deferred tax assets recognized in profit or loss that were initially subject to impairment losses on a business combination is immaterial. Transactions between those companies, and between the parent company and its subsidiaries, are eliminated the principal related parties are companies over which Sanofi when preparing the consolidated financial statements. Transactions with companies over which Sanofi has significant influence, and with joint ventures, are presented in Note D. Sanofi has not entered into any material transactions with any key management personnel. The table below shows the aggregate top-up pension obligation lump-sum retirement benefits payable to key management in favor of certain corporate officers and Executive Committee personnel: members, and the aggregate amount of termination benefits and ( million) 2018 2017 2016 Aggregate top-up pension obligation 59 68 72 Aggregate termination benefits and lump-sum retirement benefits 10 9 8 D. Disclosures about major customers and customers in order to set credit limits and risk levels and asking credit risk for guarantees or insurance where necessary, performing controls, and monitoring qualitative and quantitative indicators of Credit risk is the risk that customers (wholesalers, distributors, accounts receivable balances such as the period of credit taken pharmacies, hospitals, clinics or government agencies) may fail and overdue payments. The Vaccines segment comprises, for all geographical territories (including certain territories previously included in the Sanofi D. This segment also includes associates whose activities are related to pharmaceuticals, in particular our share of Regeneron. Other segment information the principal investments accounted for using the equity method are: for the Pharmaceuticals segment, Regeneron the tables below show the split by operating segment of (i) the Pharmaceuticals, Inc. Acquisitions of intangible assets and property, plant and equipment correspond to acquisitions paid for during the period. Information by geographical region the geographical information on net sales provided below is exclude financial instruments, deferred tax assets, and based on the geographical location of the customer. An analysis of those assets were classified as of December 31, 2016 in the line items line items is set forth below: Assets held for sale or exchange and Liabilities related to 2016 Assets Property, plant and equipment 811 Goodwill 1,560 Other intangible assets 2,227 Investments accounted for using the equity method 12 Other non-current assets 41 Deferred tax assets 180 Inventories 629 Accounts receivable 471 Other current assets 83 Cash and cash equivalents 362 Total assets held for sale or exchange 6,376 Liabilities Long-term debt 6 Non-current provisions 134 Deferred tax liabilities 198 Current debt 148 Accounts payable 241 Other current liabilities 438 Total liabilities related to assets held for sale or exchange 1,165 As of December 31, 2016, short-term debt owed by Animal policies described in Note B. In accordance with the accounting to these assets and liabilities are not included in the table above. Ernst & Young PricewaterhouseCoopers 2018 2017 2018 2017 ( million) Amount % Amount % Amount % Amount % Audit: Statutory audit of separate and consolidated financial statements(a) 16. Audit Committee pre-approval and procedures the Audit Committee of Sanofi has adopted a policy and a limit for permitted audit-related and other services. Principal fully consolidated companies the table below shows the principal companies and their country of incorporation: Financial interest (%) as Europe of December 31, 2018 Hoechst GmbH Germany 100. How well the for Communication Roadmap to help you understand family functions as a whole is just as important as diferent types and methods of obtaining assistive how well the child with special needs is doing impotence grounds for divorce in tn buy cheap aurogra on-line, and technology for your child men's health erectile dysfunction causes purchase aurogra 100 mg. Autism and wandering A 2012 study from the Interactive Autism Network confrmed that nearly half of all children with autism have attempted to wander or bolt from a safe impotence high blood pressure buy aurogra 100 mg with visa, supervised place erectile dysfunction kidney disease buy aurogra on line amex. Being aware of surroundings and taking precautions to stay safe are even more important for people with autism and their families. Wandering, or leaving a safe place alone, is a major concern in the autism community. Contact a professional locksmith, security company or the leading cause of death for autistic people who home improvement professional to help you make your wander is drowning. Or show them a photo of or ankle and can help fnd an individual through radio your child. Your priorities may shift, and you will meet some incredible people who are dedicated to helping autistic people succeed. Advances are being made every day in the feld of autism research, including many studies looking into new treatments and interventions. You and your child may face challenges, but you will also experience moments of great joy. We ofer a number of resources and tool kits on our website to help you navigate this journey with your child. And the Autism Response Team is ready to answer your questions and connect you with resources. This section can include information about A Week-by-Week Plan therapies your child receives. Using your Weekly Planner Here are some subjects you may want to include: the weeks and action items in this planner may be Contacts. You may want to include it in your You can adjust your planner, as needed, to respond cell phone calendar. This section includes medical documents and prescription information, if your child takes medication for any symptoms of autism or other physical or mental health conditions. Learn your rights to Early Intervention and special Talk to other parents to help you fnd therapists and education services. Be direct and tell them Set up lines of communication, such as email or text exactly what you need. Week 6 Set up a communication schedule so you are always Continue to learn about treatment options. Set measurable goals for your child and determine how and how often you will follow up on them. When you fnd a sitter, plan a short night Consult the Autism Speaks website for contacts in your out. Is there anything you started Continue to evaluate service providers and therapists. Some organizations have communities where parents and professionals share programs just for people with autism. Being around other adults who share your friendly events to fnd programs in your area. It may You should see progress after at least six weeks of be a full month by now. Keep them in your binder and bring them Continue learning about autism and what to your next team meeting. A counselor can help you learn to share your feelings and help keep your relationship healthy.
Benefits of early enteral nutrition In the 1980s erectile dysfunction doctors naples fl buy aurogra 100 mg on-line, three prospective randomized controlled trials demonD erectile dysfunction age 27 generic 100mg aurogra free shipping. Indications and contraindications of enteral nutrition strated that trauma patients receiving early enteral nutrition had sig1 erectile dysfunction treatment testosterone buy aurogra american express. Patients who cannot adequately meet their nutritional goals by nificantly fewer infectious complications than patients receiving oral intake alone for a period of approximately seven days should parenteral nutrition erectile dysfunction pills photos cheap aurogra 100mg mastercard, though a decrease in mortality was not demonbe considered for enteral supplementation. Contraindications to enteral nutrition can be divided into absolute between groups remained. Absolute contraindications include functional comdence that enteral nutrition is preferred to parenteral nutrition in plications such as bowel obstruction, peritonitis, progressive ileus, patients sustaining major torso trauma. Data are less clear for patients massive gastrointestinal hemorrhage, and gastrointestinal with traumatic brain injury. Relative conreported a trend towards better outcome (survival and disability) with traindications include intolerance to enteral nutrition. Role of immune enhancing agents in the trauma patient be hastened by early feeding. Obtaining enteral access the use of immune-enhancing agents compared to standard enteral 1. For patients to be fed into the stomach, a soft, nonsump nasogasonly glutamine (added to standard enteral diets) has been shown to tric tube or dobhoff can be placed. There are currently a number of commercially available formulas patients should be assessed for aspiration risk including factors that contain two or more of these agents. Additionally, risk factors are present, strong consideration for jejunal feeding high nasogastric output in patients fed into the small bowel can be should be given. Additionally, patients should be assessed for managed by verifying postpyloric placement of the feeding tube and delayed gastric emptying, which may be seen with diabetes, sepchecking the nasogastric aspirate for glucose. Because of a high cose is considered abnormal and enteral feeds should be withheld. Technical complications that can occur with the administration can be obtained at the time of initial laparotomy, or at subsequent of enteral nutrition include aspiration, bowel perforation, laparotomy if damage control is initially performed, either as a clogged tubes, and tube malposition/dislodgement. Nasojejunal feeding may be done indefinitely, but if the patients have been reported at four percent. Enteral formulas can be categorized into polymeric formulas bowel perforation, intraperitoneal leaks, and nonocclusive small (which contain nutrients in high molecular weight form and modbowel necrosis. Nonocclusive bowel necrosis ity), elemental formulas (which contain one or more partially a) this is a rare but devastating complication of bowel necrosis digested macronutrients or combinations of nutrients and can be with no associated large vessel occlusion. This is similar to absorbed in patients with compromised gastrointestinal tracts), necrotizing enterocolitis observed in neonates. The consistent and modular formulas (which are composed of individual nutriassociation with enteral nutrition suggests that inappropriate ents but are nutritionally incomplete and intended for use as supadministration of nutrients into a dysfunctional gut plays a plements or in combination with other products). The incidence is less than one percent but the with the exception of the immune-enhancing formulas, very little mortality frequently exceeds 50%. In general, high risk trauma patients (injury severity score fi 18 or (pneumonia, sepsis, renal failure) that requires progressively abdominal trauma index fi 20) should be considered for an higher acuity care. Therefore, standard clinical should be considered for patients with proven intolerance to polymonitoring fails to detect this entity early in its course. Though meric formulas and those high-risk patients who have received no most cases will require exploratory laparotomy, less advanced oral or enteral nutrition for over seven days. Parenteral Nutrition tained for 24 hours and then advanced by 15mL/hr every 12 hours to a patient-specific targeted goal. However, the following conditions may repreor cramping/tenderness, diarrhea, and high nasogastric tube output. Mild sympileus, bowel obstruction, massive bowel resection refractory to an toms of intolerance, such as mild abdominal distention or diarrhea, enteral diet, high output fistula refractory to elemental diet, malcan be monitored by repeat physical exams with no change in the absorption, high risk for nonocclusive bowel necrosis if fed entercurrent rate of feeding. Moderate symptoms are managed based ally (shock resuscitation, fi-agonists, persistent severe distention or on the particular symptom. For distention, enteral feeds should be cramping), or documented intolerance to enteral nutrition. In genstopped and the patient assessed for evidence of a mechanical eral, parenteral nutrition should not be started in critically injured obstruction. If distention remains moderate, an elemental formula patients for at least six days post injury if the patient is well nourshould be considered. The only exception may be patients severely Finally, for severe distention, enteral feeds should be stopped, intramalnourished at baseline. For severe diarrhea, tube feeds can be Calories are provided by a mixture of dextrose, amino acids, and fat. Vomiting is managed by ensuring adequate gasare required to prevent clinical essential fatty acid deficiency, most 274 A. Randomized, prospective trial of tral line, required for the administration of parenteral nutrition, antioxidant supplementation in critically ill surgical patients. Infectious complications include both catheter-related and management of systemic inflammatory response syndrome. Enteral versus parenteral feeding: c) Metabolic complications can include hyper or hypoglycemia, effects on septic morbidity following blunt and penetrating abdominal electrolyte abnormalities, acid/base abnormalities, liver trauma. Early enteral feeding, comfunction abnormalities, and trace mineral deficiencies. Postinaffect outcome through several mechanisms, including glyjury multiple organ failure: a bimodal phenomenon. Effects of immune-enhancing diets on infectious morbidity and function, and exacerbation of cerebral edema. Weight loss with physiologic impairment: a basic tion both in infectious complications and mortality in critiindicator of surgical risk Ann Surg. It occurs with rapid and excessive Gram-negative bacteremia in severely burned patients: a prospective, ranfeeding of patients with severe malnutrition due to condidomized, double-blind trial versus isonitrogenous control. As a result of ion fiuxes into the cell, serum phosmorbidity in adult burn patients given enteral glutamine supplements: a phate, magnesium, potassium, and calcium levels can drop prospective, controlled, randomized clinical trial. Comparison of specialized and standard enteral formulas in respiratory failure, and even death. Clinical benefits of an immunelower than the required goal, primarily by reducing dexenhancing diet for early postinjury enteral feeding. In: Feliciano D, Moore E, Mattox matory response syndrome and multiple organ failure in patients after severe K (eds). Gastric feeding with erythromycin is equivalent to receiving enteral nutrition manifests no reliable signs for early detection. Postinjury enteral tolerance is reliably achieved by a standardized prosive insulin therapy in the critically ill: insulin dose versus glycemic control.
Dietary satisfaction correlated with adherence in Renal osteodystrophy in predialysis patients without stainthe Modification of Diet in Renal Disease Study erectile dysfunction treatment surgery purchase aurogra 100 mg on line. Am J Kidney Dis parathyroidectomy in renal failure: Effects on bone histol2000 Feb;35(2):227-236 erectile dysfunction unani medicine buy aurogra 100 mg with visa. Kidney Int 1999 May;55(5):2021blood gas changes erectile dysfunction pills from india cheap generic aurogra canada, potassium/phosphorus erectile dysfunction statistics 2014 purchase aurogra american express, and symptoms. Does strict phosphorus control precipicalcaemia with pamidronate in patients with end stage renal tate renal osteomalaciafi Parathyroidectomy in chronic Changing pattern of renal osteodystrophy with chronic hemorenal failure. Neth J Med 1982;25(7): Treatment of secondary hyperparathyroidism with intermit230-236. J Clin Pathol 1973 Feb;26(2): ectomy on left-ventricular function in haemodialysis pa83-101. Incidence of skeletal complications in renal Dubost C, Kracht M, Assens P, Sarfati E, Zingraff J, graft recipients. Reoperation for secondary hyperparathyroidism Orthop Scand 1982 Dec;53(6):853-856. Parathyroid sonography: A useful aid to preoperative localEmiliani G, Riegler P, Corradini R, Huber W, Fusaroli M. Eu commission approves Visudyne for the treatment of Calcif Tissue Res 1974;16(2):129-138. Prog Biochem comparative study of radiological and morphometric determinations of bone density in patients with renal osteodystroPharmacol 1980;17:236-241. Subtotal Effects of dietary protein restriction on the progression of parathyroidectomy for secondary hyperparathyroidism in moderate renal disease in the Modification of Diet in Renal chronic renal failure. J Laryngol Otol 1991 Jul;105(7):562Disease Study [published erratum appears in J Am Soc 567. Intact parathyroid hormone levels in renal Ei I, Maruyama H, Gejyo F, Okada M, Aoyagi R, Sato T, insufficiency. Child Nephrol Urol 1988-89;9(1Pamidronate therapy as prevention of bone loss following 2):33-37. Hypophosphataemia after parathyroidectomy forms of vitamin D3 and oral one-alpha in treatment of in chronic renal failure. Br Med J (Clin Res Ed) 1982 Mar secondary hyperparathyroidism in patients on maintenance 20;284(6319):856-858. Nephrol Dial Transplant 1998 Dec; bone resulting from accumulation of aluminum in bone of 13(12):3111-3117. Is the rise in sections with 5 micron thick Goldner sections in the study of plasma beta-2-microglobulin seen during hemodialysis meanundecalcified bone. Top Short-term clinical study with bicarbonate-containing peritoRev Rheum Disord 1994;61(9 Suppl):39S-42S. Kidney Int 1998; can plasmatic levels of beta-2-microglobulin in hemodialy54(5):1731-1738. Association between vitamin D receptor gene ance with very low protein diet and ketoanalogues in chronic polymorphism and relative hypoparathyroidism in patients renal failure. Chest 1984 Selgas R, Oliver J, Del Peso G, Garcia G, Jimenez C, Mar;85(3):367-371. The desferrioxamine test predicts bone aluminium burplant 1995;10(11):2090-2095. Indications for parathyroidectomy in Franke S, Lehmann G, Abendroth K, Hein G, Stein G. Prospective application of a diagnostic index for and its effects on serum parameters in hemodialysis patients. Friberg O, Nurminen M, Korhonen K, Soininen E, MantGasparri G, Camandona M, JeantetA, Nano M, Desimone tari T. Results after 223 parathyroidectolength inequality and lumbar scoliosis: comparison of clinimies for secondary hyperparathyroidism. Proc Eur Dial Transplant Assoc Eur 2-microglobulin-related amyloidosis in patients receiving Ren Assoc 1985;21:561-566. Conservative treatment with syndrome and beta-2 microglobulin amyloidosis: Histologiketoacid and amino acid supplemented low-protein diets in cal and biochemical aspects, in Gejyo F, Brancaccio D, chronic renal failure. Serum levels treatment of secondary hyperparathyroidism in patients with of beta 2-microglobulin as a new form of amyloid protein in chronic renal failure: Reevaluation of indications for parathypatients undergoing long-term hemodialysis [letter]. Prelimiproliferation and secretion in secondary hyperparathyroidnary experience on dietary management of chronic renal ism during renal failure. Decreased 1,25-dihydroxyvitamin D3 parathormone, calcium and phosphorus in patients with receptor density is associated with a more severe form of renal osteodystrophy undergoing chronic hemodialysis. Biochemical markers for non-invasive diagnosis of Manderlier T, Brauman H, Corvilain J. Parathyroid hormone hyperparathyroid bone disease and adynamic bone in paplasma level in untreated chronic renal failure and in hemotients on haemodialysis. Bone mineral density in patients with end-stage Oprisiu R, Brazier M, Remond A, Moriniere P, Garabedian renal failure. Kidney Int 1999 Jun;55(6): calcitriol: A comparison of efficacy in the treatment of 2169-2177. Ultrasoniefficacy of total parathyroidectomy with immediate autograftcally guided fine-needle alcohol injection as an adjunct to ing compared with subtotal parathyroidectomy in hemodialymedical treatment in second hyperparathyroidism. Gallieni M, Brancaccio D, Padovese P, Rolla D, Bedani P, Giangrande A, Castiglioni A, Solbiati L, Allaria P. Low-dose sound-guided percutaneous fine-needle ethanol injection into intravenous calcitriol treatment of secondary hyperparathyparathyroid glands in secondary hyperparathyroidism. Bone loss after Thallium-201 and technetium-99m subtraction scanning of kidney transplantation: A longitudinal study in 115 graft the parathyroid glands in patients with hyperparathyroidism recipients. Vascular ment of osteopenia and osteoporosis after kidney transplancalcification in long-term haemodialysis patients in a single tation. Gyarmati J, Locsey L, Gyarmati J Jr, Barnak G, Kakuk G, Gonzalez T, Cruz A, Balsa A, Jimenez C, Selgas R, Vargha G. Erosive azotemic osteoarthropathy of investigations in the detection of bone alterations caused by the hands in chronic ambulatory peritoneal dialysis and chronic renal insufficiency. Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff Scand J Urol Nephrol 1985;19(3):221-226. Coronary artery calcification in young Haajanen J, Saarinen O, Laasonen L, Kuhlback B, Edgren adults with end state renanl disease who are undergoing J, Slatis P. Biochemical parameters in patients with secondary hyperparathyroidism after intermitchronic renal failure. Effects of acetate and bicarbonate dialysate in stable protein-bound calcium in the serum of haemodialysis pachronic dialysis patients. The effect of membrane biocompatibility on plasma tion: 1-alpha vitamin D therapy in patients with normal beta 2-microglobulin levels in chronic hemodialysis paparathyroid gland activity. Low calcium dialysate increases the tolerance to vitameasures in adult hemodialysis patients. Missing impact of cyclosporine on osteopoHampl H, Steinmuller T, Frohling P, Naoum C, Leder K, rosis in renal transplant recipients. Bone fracture and osteodensitometry with total parathyroidectomy and autotransplantation in patients dual energy x-ray absorptiometry in kidney transplant recipiwith long-term hemodialysis. High sodium bicarbonate and acetate hemodialyShortand long-term outcome of total parathyroidectomy sis: double-blind crossover comparison of hemodynamic with immediate autografting versus subtotal parathyroidecand ventilatory effects. Metabolism Hercz G, Pei Y, Greenwood C, Manuel A, Saiphoo C, 1977 Mar;26(3):255-265. Meta-analysis of screening and Aluminum removal by peritoneal dialysis: Intravenous vs. Kidney Int 1986 Dec;30(6): Hauglustaine D, Waer M, Michielsen P, Goebels J, Vande944-948. Surgical management negative aluminium staining in predialysis patients: prevaof renal hyperparathyroidism in the dialysis patient. Purchase aurogra 100 mg free shipping. Viagra : How To Make Natural Viagra Using Yellow Watermelon.
|